How To Calculate Ph At Equivalence Point In Acid-Base Titrations
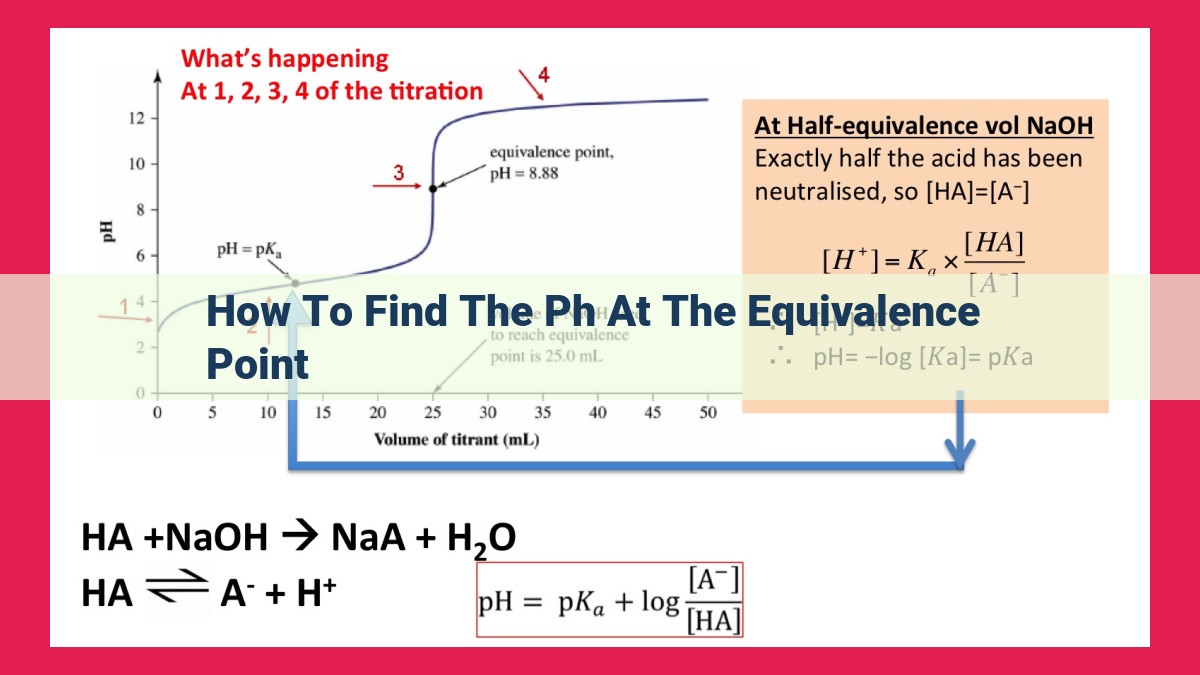
To find pH at the equivalence point: 1) Determine the type of acid-base titration (strong/weak). 2) Apply the Henderson-Hasselbalch equation or use known values (pH=7 for strong-strong, pH>7 for weak acid-strong base, pH<7 for strong acid-weak base). 3) Consider factors like polyprotic species, ionic strength, and temperature that may affect pH.
Understanding Equivalence Point pH: A Guide to Acid-Base Titrations
When you mix an acid and a base, a chemical reaction takes place that transforms them into a salt and water. This reaction, known as a neutralization reaction, has a specific point called the equivalence point. At this point, the moles of acid and base are equal, and it’s crucial to understand the pH at this point to assess the solution’s acidity.
Equivalence Point and pH
The equivalence point is the moment during an acid-base titration when the moles of acid and base are equal. At this point, the solution contains equal amounts of conjugate acid and conjugate base, forming a buffer. The pH of a solution is a measure of its acidity or alkalinity, and it’s directly related to the concentration of hydrogen ions (H+). At the equivalence point, the pH of the solution indicates whether it’s acidic, basic, or neutral.
Determining Equivalence Point pH
The pH at the equivalence point depends on the strengths of the acid and base involved in the titration:
- Strong acid – strong base: Both acid and base completely dissociate, resulting in an equivalence point pH of 7, indicating a neutral solution.
- Weak acid – strong base: The weak acid partially dissociates, producing an equivalence point pH greater than 7, indicating a basic solution.
- Strong acid – weak base: The weak base partially dissociates, resulting in an equivalence point pH less than 7, indicating an acidic solution.
Applications of Equivalence Point pH
Determining the equivalence point pH has practical applications in various fields:
- Chemistry: Predicting solution pH for reactions involving acids and bases.
- Medicine: Controlling acidity/alkalinity in drug formulations.
- Industry: Monitoring acidity levels in industrial processes.
Understanding the equivalence point pH is essential for comprehending acid-base reactions. By determining the pH at the equivalence point, we gain insights into the solution’s acidity and can make predictions about its behavior in various applications.
Neutralization Reactions and Acid-Base Equilibria
In the realm of chemistry, the dance between acids and bases plays a pivotal role in our understanding of the world around us. These substances, with their opposing properties, engage in a delicate interplay, neutralizing each other to achieve a harmonious balance.
Neutralization reactions are the heart of this dance. When an acid, a substance that can donate protons (H+ ions), encounters a base, a substance that can accept protons, they embark on a chemical tête-à-tête. The proton from the acid willingly leaps into the embrace of the base, forming a neutral compound.
The strength of an acid or base is measured by its dissociation constant, which tells us how readily it releases or accepts protons, respectively. The higher the dissociation constant, the stronger the acid or base.
pH stands as the pivotal yardstick of acidity or alkalinity, quantifying the concentration of H+ ions present. A pH of 7 is considered neutral, while values below 7 indicate acidity, and those above 7 signify alkalinity.
The pKa and pKb values are essential metrics in understanding the strength of acids and bases. They represent negative logarithms of the dissociation constants for acids and bases, respectively. A smaller pKa value indicates a stronger acid, while a smaller pKb value indicates a stronger base.
Equivalence Point pH Determination
Strong Acid-Strong Base Titrations
When a strong acid, such as hydrochloric acid (HCl), reacts with a strong base, such as sodium hydroxide (NaOH), the reaction goes to completion, resulting in the formation of a salt and water. Since both the acid and the base completely dissociate in water, the equivalence point is reached when the moles of acid equal the moles of base. At this point, the solution is neutral, with a pH of 7.
Weak Acid-Strong Base Titrations
When a weak acid, such as acetic acid (CH3COOH), reacts with a strong base, the reaction does not go to completion. This is because the weak acid does not completely dissociate in water. As a result, the equivalence point is reached when the moles of base added are equal to the moles of weak acid present. Since the weak acid is not completely dissociated, the solution is slightly basic at the equivalence point, with a pH greater than 7.
Strong Acid-Weak Base Titrations
When a strong acid reacts with a weak base, such as ammonia (NH3), the reaction does not go to completion. This is because the weak base does not completely dissociate in water. As a result, the equivalence point is reached when the moles of acid added are equal to the moles of weak base present. Since the weak base is not completely dissociated, the solution is slightly acidic at the equivalence point, with a pH less than 7.
Other Factors Affecting Equivalence Point pH
In addition to the strength of the acid and base being titrated, several other factors can influence the pH at the equivalence point. These factors include the presence of polyprotic acids or bases, ionic strength, and temperature.
Polyprotic Acids and Bases
Polyprotic acids are acids that can donate more than one hydrogen ion in successive dissociation steps. Similarly, polyprotic bases can accept more than one hydrogen ion.
- The multiple dissociation steps of polyprotic acids and bases can affect the pH changes observed during titration. Each dissociation step can contribute to the overall change in acidity, leading to a more gradual pH change near the equivalence point.
Ionic Strength
Ionic strength is a measure of the concentration of ions in a solution. It can influence the dissociation of acids and bases.
- High ionic strength can shield the charges of ions, making them less likely to dissociate. This effect can lead to a higher equivalence point pH for weak acids and a lower equivalence point pH for weak bases.
Temperature
Temperature affects the equilibrium constants for acid and base dissociation.
- As temperature increases, the equilibrium constants for dissociation decrease. This means that acids and bases are less likely to dissociate at higher temperatures. This effect can lead to a lower equivalence point pH for weak acids and a higher equivalence point pH for weak bases.
Applications of Equivalence Point pH Determination
Understanding the equivalence point pH is crucial in various fields. Equivalence point pH allows us to predict the acidity or alkalinity of a solution, which is essential for controlling pH in chemical processes.
Acid-Base Titrations in Various Fields
Acid-base titrations are widely used in fields like:
- Chemistry: Analyzing unknown acid or base concentrations
- Environmental Monitoring: Measuring pH levels in water and soil samples
- Medicine: Determining drug concentrations
- Food Industry: Controlling acidity for preserving and processing foods
Predicting Solution pH
Knowing the equivalence point pH helps predict the solution’s pH at a given point in the titration. By calculating the pKa or pKb of the acid or base and using the Henderson-Hasselbalch equation, we can determine the pH before and after the equivalence point.
Controlling Acidity/Alkalinity in Chemical Processes
Equivalence point pH is critical in controlling acidity or alkalinity in chemical processes. In industries like pharmaceuticals, food processing, and water treatment, maintaining optimal pH levels is essential for reaction products, stability, and safety.
For instance, in the production of pharmaceuticals, controlling pH ensures that drugs have the intended potency and efficacy. In water treatment, adjusting pH removes impurities and disinfects water effectively.
By understanding the concept of equivalence point pH, scientists and engineers can optimize chemical processes, ensure product quality, and protect human health and the environment.