Understanding Buffers: Their Role In Ph Regulation And Applications
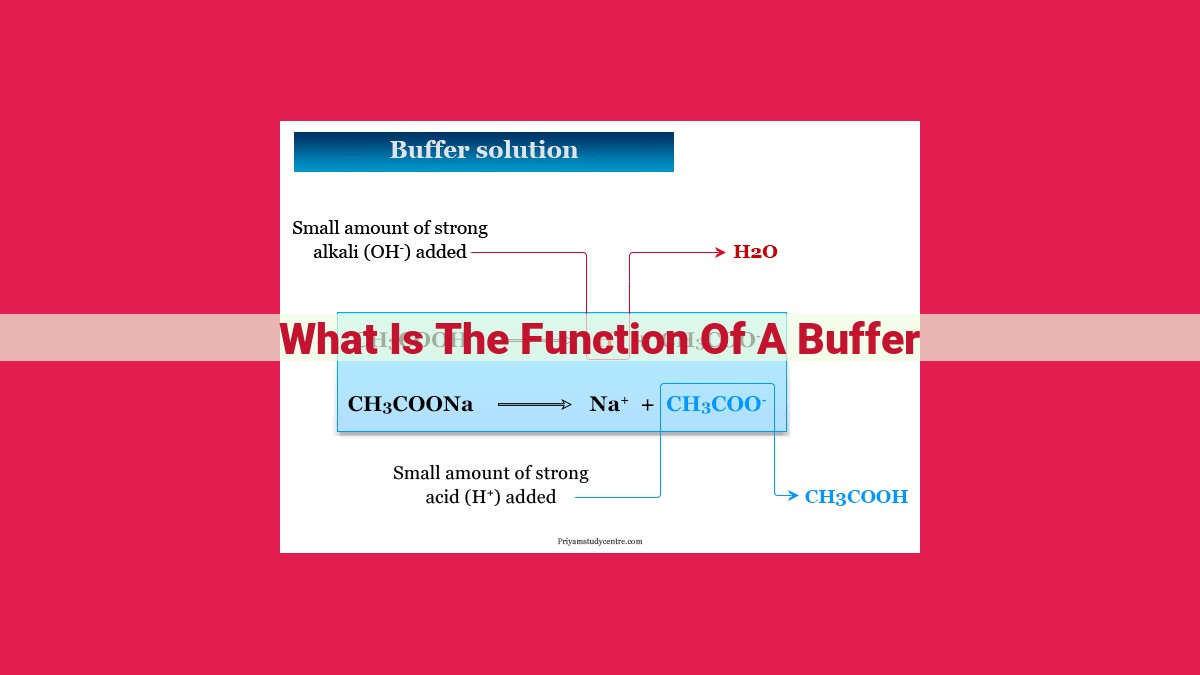
A buffer is a solution that resists changes in pH when acids or bases are added. It consists of a weak acid and its conjugate base, or a weak base and its conjugate acid. Buffers maintain a narrow pH range through acid-base equilibria, and their capacity to do so is determined by the dissociation constant of the weak acid or base. The composition of buffers is crucial, with the Henderson-Hasselbalch equation used to calculate pH based on component concentrations. Buffers play a vital role in biological systems for pH balance, industrial processes for pH control, and analytical chemistry for acid-base titrations and pH measurements.
Buffers: The Unsung Heroes of pH Regulation
In the realm of chemistry, there exist unsung heroes that silently work to maintain the delicate balance of life: buffers. These remarkable chemical solutions play a crucial role in regulating pH, the measure of acidity or alkalinity of a substance.
Imagine your body as a finely tuned orchestra, where every instrument must play in harmony. Just as a conductor ensures the cohesion of the orchestra, buffers act as conductors within our cells, keeping the pH within a narrow range essential for life.
Without buffers, our bodies would be vulnerable to the harsh effects of acids and bases, which could disrupt cellular processes and wreak havoc on our overall well-being. Buffers stabilize pH, preventing extreme fluctuations that can lead to cell damage or even organ failure.
pH Regulation with Buffers: A Guardian of pH Stability
Buffers: The Unsung Heroes of pH Control
In the complex realm of chemistry, pH plays a crucial role in the behavior of substances. To maintain a specific pH, scientists rely on buffers, chemical guardians that prevent drastic pH fluctuations. But what exactly are buffers, and how do they achieve this remarkable feat?
Acid-Base Equilibria: The Dance of Ions
Buffers operate based on the principles of acid-base equilibria. In aqueous solutions, acids release hydrogen ions (H+), while bases release hydroxide ions (OH-). When a buffer is added to a solution, its component acid and base undergo a balancing act. The acid dissociates, releasing H+ ions, while the base accepts H+ ions to form conjugate acid-base pairs. This equilibrium ensures that the pH remains within a narrow range.
Dissociation Constants: The Measure of Buffer Strength
The dissociation constant (Ka) of an acid quantifies its tendency to dissociate. A strong acid has a large Ka, indicating significant dissociation and high H+ ion concentration. Conversely, a weak acid has a small Ka, meaning it remains largely undissociated. The Ka value of a buffer’s acid determines its buffer capacity, which is its ability to resist pH changes.
Buffer Capacity: The Shield against pH Fluctuations
Buffer capacity is the key to maintaining a stable pH. Buffers with a large buffer capacity can absorb significant amounts of acid or base without undergoing significant pH changes. This is because the dissociation of the acid and recombination of the conjugate base compensate for the added ions, preventing drastic swings in H+ ion concentration.
Titration Curves: Mapping pH Changes
Titration curves provide a graphical representation of pH changes during acid-base reactions. When a strong acid is added to a buffer solution, the pH initially decreases rapidly but then plateaus at the buffer’s pKa. This pKa is the pH at which the acid and conjugate base concentrations are equal. The plateau indicates that the buffer is effectively neutralizing the added acid, maintaining a relatively stable pH.
Buffer Capacity and Titration Curves: Unlocking pH Stability and Reactivity
In the world of chemistry, buffers play a crucial role in maintaining a stable pH even in the face of acid or base additions. This remarkable ability, termed buffer capacity, allows buffers to effectively resist pH changes and ensure the smooth functioning of various biochemical and industrial processes.
Titration curves, on the other hand, provide a graphical representation of the pH changes that occur during acid-base reactions. By analyzing these curves, scientists can determine the buffer capacity of a solution and gain insights into its ability to neutralize acids or bases.
Buffer Capacity: The Key to Stability
Buffer capacity is a measure of a buffer’s ability to resist changes in pH when an acid or base is added. The greater the buffer capacity, the more effectively the buffer can maintain a constant pH. This is especially important in biological systems, where even slight pH variations can have profound effects on cellular processes.
The buffer capacity of a solution is directly related to the concentration of the buffer components. A buffer with higher concentrations of its components will have a greater buffer capacity, as it contains more ions available to neutralize added acids or bases.
Titration Curves: Unveiling Buffer Behavior
Titration curves are plots that show the change in pH of a solution as an acid or base is gradually added. These curves provide valuable information about the buffering capacity of a solution.
The initial pH of the solution indicates the strength of the buffer. A higher initial pH indicates a stronger buffer, as it can neutralize more acid before a significant pH change occurs. The shape of the curve, especially the slope, also reveals the buffer capacity. A steeper slope indicates a weaker buffer, as the pH changes more rapidly upon the addition of acid or base.
Understanding Titration Curves
- Steep regions of a titration curve represent areas where the buffer is overwhelmed by the added acid or base, leading to a rapid change in pH.
- Flat regions indicate that the buffer is effectively neutralizing the added acid or base, maintaining a relatively constant pH.
- The equivalence point is the point in the titration where the buffer capacity is at its lowest. At this point, the buffer has been completely neutralized, and any further addition of acid or base will cause a significant pH change.
Applications of Buffer Capacity
Buffer capacity is essential in numerous applications, including:
- Biological systems: Buffers regulate pH in body fluids, such as blood, to maintain optimal enzyme function.
- Industrial processes: Buffers control pH in manufacturing processes, ensuring product quality and efficiency.
- Analytical chemistry: Buffers are used in acid-base titrations to determine the concentration of unknown acids or bases.
Understanding the Composition and pH Calculations of Buffers
Defining Buffers: The pH Guardians
Buffers are like the unseen heroes of chemical reactions, silently working to maintain a stable pH even when faced with acid or base challenges. They do this by countering pH shifts, ensuring a narrow and optimal pH range for crucial chemical processes.
Components of a Buffer: The Weak Acid and Its Conjugate Base
At the heart of a buffer lie two crucial components: a weak acid and its conjugate base. A weak acid is an acid that partially dissociates in water, creating a small concentration of hydrogen ions (H+). Its conjugate base, on the other hand, is the base formed when the weak acid donates a proton (H+).
Working Together: The Acid-Base Conjugate Pair
This acid-base conjugate pair plays a dynamic role in buffering. When excess H+ ions enter the solution due to an acid addition, the conjugate base neutralizes them, using its spare protons to form the weak acid. Conversely, if OH- ions (hydroxide ions) enter, the weak acid neutralizes them, using its protons to form the conjugate base.
The Henderson-Hasselbalch Equation: Calculating Buffer pH
With knowledge of the weak acid and conjugate base concentrations, we can calculate the pH of the buffer using the Henderson-Hasselbalch equation:
pH = pKa + log([A-] / [HA])
Here, pKa is the acid dissociation constant for the weak acid, [A-] is the concentration of the conjugate base, and [HA] is the concentration of the weak acid.
Buffer Optimization: Balancing Components
The capacity of a buffer to resist pH changes depends on component concentrations and their ratio. A higher concentration of either the weak acid or conjugate base enhances buffering capacity.
Fine-Tuning pH: Adjusting Component Ratios
To adjust the buffer’s pH, we can manipulate the component ratio. By adding more weak acid, we lower the pH, while adding more conjugate base raises the pH. This pH adjustment allows us to tailor buffers to specific applications.
Neutralization Reactions in Buffers
In the realm of chemistry, buffers play a crucial role in maintaining the pH balance of solutions. These clever chemical concoctions act like pH guardians, ensuring that pH fluctuations are kept to a minimum. When acids and bases encounter buffers, something fascinating happens – a neutralization reaction. Let’s delve into this chemical dance and uncover its secrets.
Neutralization reactions are chemical reactions in which acids and bases react to form salts and water, releasing heat in the process. The reaction can be represented as:
Acid + Base → Salt + Water
When an acid and a base react in a buffer solution, they effectively neutralize each other’s effects. The buffer acts as a mediator, mitigating the drastic pH changes that would otherwise occur. This is because buffers contain both a weak acid and its conjugate base, which work together to absorb hydrogen ions (H+) or hydroxide ions (OH-) when added to the solution.
The concept of equivalents is key in understanding neutralization reactions. An equivalent is the amount of an acid or base that can donate or accept one mole of H+ ions. When the number of equivalents of acid added to a buffer solution is equal to the number of equivalents of base present in the buffer, complete neutralization occurs, resulting in a solution with a neutral pH of 7.
In practice, buffers are designed to have a specific pH and buffer capacity, which determines their ability to resist pH changes. By carefully choosing the weak acid and its conjugate base, scientists can create buffers that are tailored to specific pH ranges and applications. These buffers play a vital role in various fields, including biology, industry, and analytical chemistry, where maintaining pH balance is paramount.
The Common Ion Effect: pH Minimization in Buffers
Buffers are superheroes in the world of chemistry, safeguarding pH stability like valiant guardians. But what happens when a common ion crashes the party? Let’s dive into the fascinating interplay of common ion effect and pH minimization in buffers.
The common ion effect is like a mischievous prankster, subtly altering the pH dance. When a common ion, one that shares a charge with the buffer’s acid or base, is introduced, it plays a sneaky game with the equilibrium. Imagine a crowd of weak acid molecules, minding their own business, dissociating into ions. Now, if we toss in a bunch of common ions, they’re like, “Whoa, what’s going on?” and start rushing to reform their undissociated acid molecules.
This Le Chatelier’s principle magic tricks the buffer into thinking there are fewer ions floating around, prompting it to dissociate more acid molecules to restore the balance. As a result, the pH shift caused by the added acid or base is minimized. It’s like a buffer’s superpower: it can shrug off pH changes like a champ.
Factors Affecting Buffering Capacity
Buffering Capacity: The Unsung Hero
Buffers, the unsung heroes of pH regulation, play a crucial role in maintaining the delicate balance of acidity and alkalinity in various biological and chemical systems. Understanding the factors that affect their buffering capacity is essential to optimize their performance.
Concentration: The Key to Success
The concentration of buffer components, both the weak acid and its conjugate base, directly influences buffering capacity. Higher concentrations result in greater buffering capacity, as there are more ions available to neutralize added acids or bases. This translates into a smaller change in pH upon acid or base addition.
Ratio: The Power of Balance
The ratio of the weak acid to its conjugate base also plays a critical role. A balanced ratio ensures that the buffer has an optimal capacity to neutralize both acids and bases. When the ratio is skewed towards the weak acid, the buffer will be more effective at neutralizing bases, while a higher ratio of conjugate base will enhance its capacity for neutralizing acids.
pH Adjustment: The Fine-tuning Touch
pH adjustment techniques offer a way to optimize buffer performance. By adding small amounts of strong acid or base, the pH of the buffer can be shifted to a desired range. This is particularly useful when working with buffers that require a specific pH for their optimal function.
By considering the concentration, ratio, and pH adjustment techniques, we can harness the full potential of buffers to maintain pH stability in various applications. From biological systems to industrial processes, buffers play an indispensable role in ensuring the proper functioning of numerous reactions and processes. Understanding these factors allows us to tailor buffers to meet specific needs, maximizing their effectiveness and achieving optimal pH control.
Applications of Buffers
In the world of pH regulation, buffers play a crucial role in maintaining a stable environment in various settings. Beyond their fundamental importance in biological systems, buffers find widespread applications in industry and analytical chemistry.
Biological Applications
Maintaining pH Balance: In our bodies, buffers work tirelessly to stabilize pH levels in blood, digestive fluids, and cellular compartments. This delicate balance is essential for a wide range of physiological processes, including enzyme activity, nerve transmission, and muscle function.
Industrial Uses
In the realm of industry, buffers play a critical role in regulating pH during manufacturing processes. From pharmaceuticals to textiles and food processing, buffers ensure optimal conditions for chemical reactions, preventing unwanted side reactions and protecting equipment from corrosion.
Analytical Chemistry
In the laboratory, buffers are indispensable tools for acid-base titrations and pH measurements. They provide a stable medium, allowing for accurate determination of acid or base concentrations. By minimizing pH changes, buffers ensure reliable and precise results.
In summary, buffers are essential in maintaining pH stability across diverse applications. From the human body to industrial settings and analytical laboratories, they play a vital role in ensuring optimal conditions for a wide range of chemical processes and physiological functions.