Buffer Solutions: Essential For Ph Stability In Biological Systems
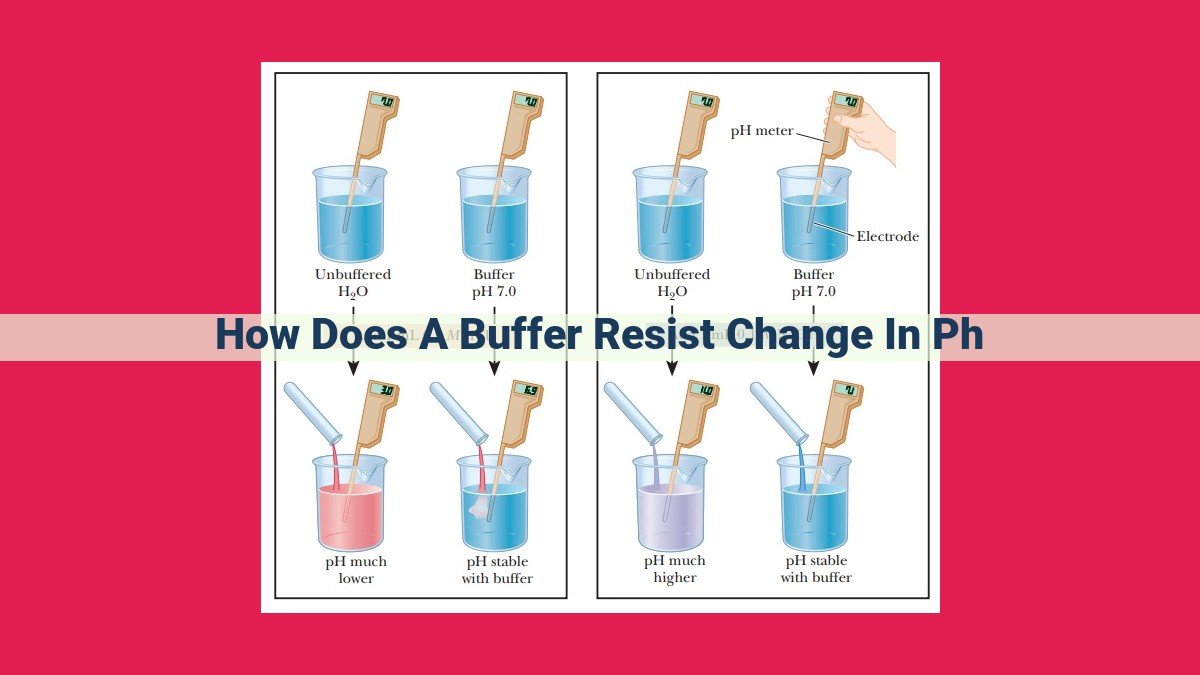
Buffers resist pH changes by maintaining a stable hydrogen ion concentration. They contain a weak acid that dissociates slightly, providing a reservoir of hydrogen ions. When a small amount of acid is added, the weak acid donates protons, limiting the pH decrease. Conversely, when base is added, the weak acid’s conjugate base reacts with the excess protons, preventing the pH from rising significantly. The buffer capacity, a measure of the buffer’s resistance to pH change, depends on the concentration and dissociation constant of the weak acid. Buffers play a crucial role in biological systems, maintaining a suitable pH for enzyme function and protecting organisms from pH fluctuations.
Buffers: Maintaining the Delicate Equilibrium of Our World
In the bustling symphony of life, from the depths of our oceans to the hum of our industries, there exists an unseen yet crucial player: buffers. These chemical guardians stand as sentinels of stability, safeguarding against drastic pH shifts and protecting the delicate balance that sustains life and industry.
Buffers, the unsung heroes of chemistry, come into play whenever a system faces the threat of pH fluctuations. They act as guardians, neutralizing invading acids or bases while maintaining a remarkably steady pH. This extraordinary ability makes buffers indispensable in a wide array of fields, from biological processes to industrial applications.
But what exactly defines a buffer? Simply put, a buffer is a solution that resists changes in pH upon the addition of small amounts of acid or base. This resilient behavior stems from the presence of a weak acid and its conjugate base, which form a partnership to counterbalance pH changes. The weak acid heroically donates protons, while the conjugate base villainously accepts them, effectively neutralizing the threat and preserving the pH equilibrium.
Unveiling the Secrets of Weak Acids and Conjugate Bases: Guardians of Buffering Capacity
In the vibrant realm of chemistry, understanding the nature of weak acids and their inseparable partners, conjugate bases, holds the key to unlocking the mysteries of buffering capacity. These remarkable substances play a pivotal role in maintaining pH stability, a crucial factor in numerous biological and industrial processes.
The Essence of Weak Acids and Conjugate Bases
Weak acids, as their name suggests, only partially dissociate in water, releasing a limited number of hydrogen ions (H+). However, this partial dissociation creates a unique dance between the weak acid and its conjugate base, a species that forms by accepting the released H+.
For instance, consider the weak acid acetic acid (CH3COOH). In water, it dissociates partially to form its conjugate base, acetate (CH3COO-), and H+. The extent of this dissociation is governed by the acid dissociation constant (Ka), a measure of the acid’s strength.
Their Role in Buffering Capacity
The buffering capacity of a solution, its ability to resist pH changes, stems from the presence of these weak acids and conjugate bases. When a small amount of acid is added to a buffer solution, the conjugate base reacts with the incoming H+ ions, effectively neutralizing their effect on pH.
Conversely, if a small amount of base is added, the weak acid donates H+ ions to counteract the increase in pH. This delicate balance between dissociation and reaction maintains a relatively stable pH within a specific range.
Applications in the Biological and Industrial Worlds
Buffers find widespread application in both biological and industrial settings. In biological systems, buffers regulate pH levels within cells, ensuring optimal conditions for enzymatic reactions. In industrial processes, buffers are used to maintain specific pH levels for optimal product quality or efficiency in chemical reactions.
Understanding the properties and behavior of weak acids and conjugate bases is essential for grasping the concept of buffering capacity. These substances play a vital role in maintaining pH stability, making them indispensable tools in the fields of chemistry, biology, and industry. By unraveling the secrets of these fascinating compounds, we gain a deeper appreciation for the delicate balance that ensures stable environments for countless processes.
Dissociation of Weak Acids in Water
In the realm of chemistry, understanding the behavior of acids and bases is fundamental. Buffers, guardians of pH stability, rely heavily on the dissociation of weak acids. In this article, we embark on a journey to unravel the process of weak acid dissociation in water, a crucial concept that underpins the remarkable buffering capacity of these solutions.
When a weak acid, such as acetic acid (CH3COOH), dissolves in water, it undergoes a process called dissociation. During this transformation, a proton (H+) is released, forming its conjugate base, acetate ion (CH3COO-). This dissociation is represented by the following equilibrium reaction:
CH3COOH(aq) + H2O(l) ⇌ H3O+(aq) + CH3COO-(aq)
The extent to which a weak acid dissociates is governed by its equilibrium constant (Ka). Ka is a quantitative measure of the acid strength, indicating the tendency of the acid to donate protons. Higher Ka values correspond to stronger acids that dissociate more readily, while lower Ka values indicate weaker acids that dissociate to a lesser extent.
The equilibrium constant for acid dissociation has a profound impact on buffer behavior. Buffers are solutions that resist significant pH changes upon the addition of small amounts of acid or base. The effectiveness of a buffer is directly related to the Ka value of its weak acid component. Weak acids with lower Ka values generally produce more effective buffers because they dissociate less extensively, releasing fewer protons into the solution.
By understanding the process of weak acid dissociation and the role of equilibrium constants, we gain invaluable insights into the behavior and applications of buffers. These solutions are indispensable in numerous fields, including biological systems, analytical chemistry, and industrial processes, where pH stability is paramount.
The Common Ion Effect: A Buffer’s Stealthy Influence
Imagine a bustling market where vendors of weak acids and their conjugate bases engage in a delicate dance. The presence of these vendors, akin to common ions, sends ripples through the market, subtly altering the behavior of their acidic counterparts. This phenomenon, aptly named the Common Ion Effect, holds sway over the very essence of buffers.
The Common Ion Effect manifests when an acid dissociation reaction occurs in the presence of its own conjugate base. Consider the dissociation of acetic acid (CH3COOH) in water. In a typical scenario, a small fraction of acetic acid molecules shed their protons, leaving behind acetate ions (CH3COO-). However, if a significant amount of acetate ions is already present in the solution, it’s as if the market is oversaturated with vendors selling conjugate bases.
In such a situation, the dissociation of acetic acid is effectively suppressed. The presence of excess conjugate base shifts the equilibrium towards the undissociated form of acetic acid. This is because the conjugate base competes with the water molecules for the proton, reducing the chances of acid dissociation.
The Common Ion Effect has profound implications for buffer capacity. Buffers are solutions that resist changes in pH when small amounts of acid or base are added. The Common Ion Effect can enhance or diminish buffer capacity depending on the relative concentrations of the weak acid and its conjugate base.
If the conjugate base concentration is high, the Common Ion Effect reduces the buffer’s ability to neutralize added acid. This is because the excess conjugate base intercepts the proton before it has a chance to react with the weak acid. As a result, the buffer’s capacity to resist pH changes is compromised.
Conversely, if the conjugate base concentration is low, the Common Ion Effect has minimal impact on buffer capacity. In this scenario, the weak acid dissociates freely, and the buffer is better equipped to handle added acid or base without significant pH fluctuations.
Dilution Effects on Buffers
Buffers are solutions that resist changes in pH when small amounts of acid or base are added. They play a crucial role in various biological and industrial processes where maintaining a stable pH is essential.
Effects of Dilution on Buffer Capacity
When a buffer solution is diluted, its buffer capacity decreases. This means that it becomes less resistant to pH changes upon the addition of an acid or base. Dilution reduces the concentration of both the weak acid and its conjugate base in the solution.
Understanding Buffer Capacity
Buffer capacity is the ability of a buffer to resist pH changes. It depends on the concentration of the weak acid and its conjugate base. A higher concentration of both components results in a higher buffer capacity.
Impact of Dilution
Upon dilution, the concentration of the acid and its conjugate base decreases. This directly reduces the buffer capacity of the solution. As a result, the buffer becomes less effective in resisting pH changes.
Implications for Buffer Applications
The dilution effect on buffer capacity is an important consideration in practical applications. For example, in biological systems, buffers are used to maintain a stable pH environment for cellular processes. Dilution can impair the buffer’s ability to perform this function effectively.
Similarly, in industrial settings, buffers are used to control pH in various reactions. Dilution of a buffer can necessitate the addition of more buffer solution to maintain the desired pH range.
Dilution of a buffer solution decreases its buffer capacity. This is due to the reduction in the concentration of the weak acid and its conjugate base. Understanding the effects of dilution is important for optimizing buffer performance in various applications.
How Buffers Respond to Acid or Base Addition
When you add a small amount of acid to a buffer solution, the buffer system immediately goes into action. The weak acid in the buffer donates protons (H+) to the solution, neutralizing the added acid. This prevents the pH of the solution from changing significantly.
Similarly, when you add a small amount of base to a buffer solution, the buffer system absorbs the excess hydroxide ions (OH-), neutralizing the added base. Again, this prevents the pH of the solution from changing significantly.
The remarkable ability of buffers to resist pH changes is due to the presence of both a weak acid and its conjugate base. When acid is added, the conjugate base neutralizes it; when base is added, the weak acid neutralizes it. This buffering action is essential for maintaining a stable pH in many biological and industrial processes.
For example, in the human body, buffers help to maintain a relatively constant pH in the blood, despite the constant production of acids and bases during metabolism. Buffers are also used in industrial processes to control pH levels in reactions, ensuring optimal conditions for chemical reactions.
Buffer Capacity: A Measure of Resistance
In the realm of chemistry, buffers reign supreme as guardians of stability. These remarkable substances possess the ability to resist changes in pH when faced with the onslaught of acids or bases. Their strength in this battle is quantified by a measure known as buffer capacity.
Defining Buffer Capacity
Buffer capacity is a quantitative indicator of a buffer’s ability to neutralize added acid or base without undergoing significant pH changes. It represents the amount of acid or base that can be added to a buffer solution before a noticeable shift in pH occurs.
Factors Affecting Buffer Capacity
The buffer capacity of a solution is influenced by two primary factors:
- Buffer Concentration: Higher concentrations of buffer components (weak acid and conjugate base) result in stronger buffer capacity.
- Nature of the Weak Acid: Buffers containing weak acids with smaller dissociation constants (Ka) exhibit higher buffer capacity.
Applications of Buffers
Buff