Bromine: Exploring Valence Electrons, Covalent Bonding, And Molecular Orbital Theory

Bromine, an element with the atomic number 35, possesses 7 valence electrons. These electrons reside in the outermost energy level of the bromine atom and play a crucial role in forming chemical bonds. In a bromine molecule (Br2), the valence electrons participate in covalent bonding, sharing electron pairs to achieve a stable electron configuration. Molecular Orbital Theory explains the formation and properties of these molecular orbitals, which determine the reactivity and behavior of bromine in chemical reactions.
Understanding Valence Electrons: The Key to Chemical Bonding
In the captivating world of chemistry, we embark on a journey into the fascinating realm of valence electrons – the pivotal players responsible for shaping the dance of atoms and molecules. These dynamic electrons exist on the outermost energy level of an atom, eager to engage in thrilling interactions that forge the very fabric of our universe.
The Significance of Valence Electrons
Valence electrons hold immense significance in the chemical world. Their presence or absence, their number and arrangement, govern the chemical properties and behavior of elements. Like skilled dancers performing a mesmerizing ballet, valence electrons engage in graceful exchanges or intimate sharing, creating the captivating spectacle of chemical bonds that ultimately give rise to the boundless diversity of substances around us.
The Enchanting Dance of Br2: Unveiling the Secrets of a Diatomic Molecule
Imagine tiny, energetic beings known as valence electrons, swirling around atoms like electrons at a dance party. These electrons play a crucial role in determining how our world works. They are the ones responsible for chemical reactions, bonding, and the formation of molecules – the building blocks of all matter.
Meet Br2: A Tale of Two Bromine Atoms
Let’s dive into the world of diatomic molecules by introducing Br2, a molecule composed of two bromine atoms. Picture these bromine atoms as two elegant dancers, each with seven valence electrons ready to mingle.
The Dance of Covalent Bonding
As the bromine atoms sway and twirl, they discover a deep attraction for each other. This attraction stems from their valence electrons, eager to pair up like perfect dance partners. This dance of covalent bonding involves the sharing of electron pairs, creating a strong bond between the atoms.
Introducing Molecular Orbital Theory: The Choreography of Bonding
To fully understand the intricate dance of Br2, we turn to Molecular Orbital Theory (MOT). This theory reveals that the valence electrons of the bromine atoms don’t simply pair up; instead, they form molecular orbitals, which are regions where the electrons are most likely to be found. These orbitals can be visualized as dance floors, where the electrons move in a mesmerizing rhythm.
Dive Deeper into the World of Br2
Unveiling the Electron Configuration of Bromine
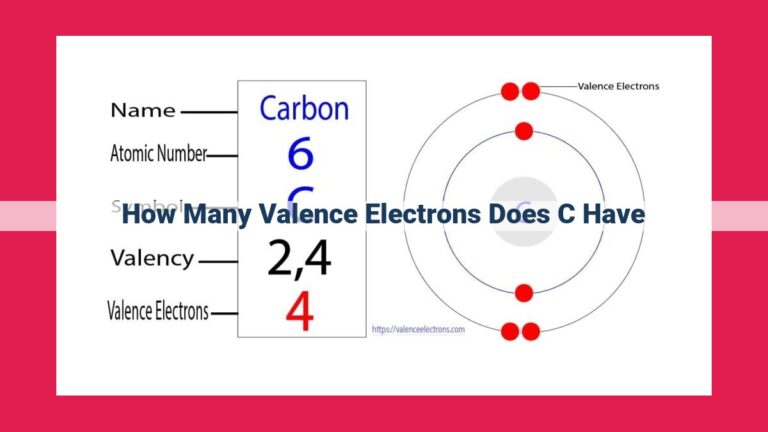
Each bromine atom possesses a unique electron configuration, which describes the arrangement of its valence electrons. This configuration, denoted as 1s²2s²2p⁶3s²3p⁵, tells us that bromine has seven valence electrons, the key players in its chemical interactions.
The Significance of Atomic Number
Every atom has an atomic number, which represents the number of positively charged protons in its nucleus. Bromine’s atomic number is 35, indicating that it has 35 protons and 35 electrons. This knowledge helps us understand the atom’s overall electrical neutrality and its ability to form stable molecules.
Valence Electrons in a Bromine Atom: Unraveling the Essence of Chemical Bonding
In the captivating realm of chemistry, valence electrons reign supreme, playing a pivotal role in shaping the interactions between atoms. Imagine a tiny stage where these electrons dance around the atomic nucleus, eagerly participating in the intricate play of chemical bonding.
Bromine, an element with an atomic number of 35, proudly boasts 7 valence electrons. To truly understand the significance of these electrons, let’s embark on a journey into the fascinating world of electron configuration.
Every atom possesses a unique arrangement of electrons, like a symphony of notes that creates a distinctive melody. This arrangement is known as its electron configuration, and it dictates the number of valence electrons an atom has. For bromine, its electron configuration is:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁵
Electron configuration helps us determine the number of valence electrons by identifying the electrons in the outermost shell. For bromine, these are the five electrons in the 4p orbital. These valence electrons are the key players in chemical bonding, eagerly seeking out partners to form stable bonds.
Understanding valence electrons is not just an abstract concept; it has real-world implications. In the case of bromine, its valence electrons explain its role as a disinfectant. Bromine’s ability to form bonds with other elements, such as hydrogen, creates compounds like hydrogen bromide (HBr), which is a powerful disinfectant used in water treatment.
In conclusion, valence electrons are the driving force behind chemical bonding, shaping the behavior of atoms and the formation of molecules. By understanding valence electrons, we unravel the secrets of chemical reactions and gain insights into the dynamic world of matter.
Atomic Number of Bromine: A Key to Understanding its Valence Electrons
In the intriguing realm of chemistry, where matter unravels its secrets, understanding the atomic number of elements is crucial. Let’s explore the atomic number of bromine and unravel its profound impact on the element’s chemical behavior.
Bromine: A Chemical Enigma
Bromine, adorned with the chemical symbol Br, is an element that captivates scientists with its fascinating properties. Residing in the halogen group, bromine exhibits a rich chemistry that stems from its unique atomic number.
Atomic Number: The Key to the Element’s Identity
The atomic number of an element, represented as Z, refers to the number of protons residing within its nucleus. This number is uniquely characteristic of each element and defines its identity. In the case of bromine, its atomic number is 35. This means that every bromine atom possesses 35 protons in its nucleus.
Impact on Valence Electrons
The atomic number plays a pivotal role in determining an element’s valence electrons. Valence electrons are the electrons occupying the outermost energy level of an atom, dictating its chemical reactivity. In the periodic table, the group number of an element aligns with the number of valence electrons it possesses. Bromine, belonging to Group 17, has seven valence electrons.
A Gateway to Understanding Bromine’s Reactivity
Understanding the atomic number and valence electrons of bromine provides a gateway to comprehending its chemical behavior. These concepts are essential for deciphering bromine’s ability to form chemical bonds, shape molecular structures, and determine its overall reactivity.
In forthcoming sections, we will delve deeper into bromine’s valence electrons, unraveling their significance in covalent bond formation, molecular orbital theory, and the Lewis structure of the Br2 molecule. Together, we will explore the fascinating world of bromine and appreciate the profound impact of its atomic number on its chemical nature.
Delving into the Electron Configuration of Bromine: Unraveling the Secrets of Br
As we embark on a journey into the fascinating world of chemistry, let’s delve into the intricate details of the electron configuration of bromine (Br). But before we dive right in, let’s revisit some fundamental concepts to set the stage for our exploration.
Atomic Orbitals: The Building Blocks of Electron Distribution
Electrons, the tiny particles that orbit the nucleus of an atom, reside in specific regions called atomic orbitals. These orbitals are like energy shells, each holding a certain number of electrons. There are four types of atomic orbitals, denoted by the letters s, p, d, and f, each with its unique shape and energy level.
Quantum Numbers: The Guiding Compass of Electron Behavior
Every electron is characterized by a set of four quantum numbers: principal quantum number (n), angular momentum quantum number (l), magnetic quantum number (ml), and spin quantum number (ms). These numbers dictate the electron’s energy, shape, orientation, and spin, respectively.
Unveiling Bromine’s Electron Configuration
Bromine, with an atomic number of 35, has an electron configuration of [Ar] 4s² 4p⁵. This means that it has 35 electrons, with the first 18 arranged in the same way as argon gas (Ar). The remaining 17 electrons occupy the fourth energy level, distributed as two in the 4s orbital and five in the 4p orbital.
Identifying the Valence Electrons: The Key Players
The valence electrons are the electrons in the outermost energy level of an atom. In the case of bromine, these are the two electrons in the 4p orbital. Valence electrons are crucial in determining an element’s chemical properties and reactivity, as they participate in bonding and interactions with other atoms.
In summary, the electron configuration of bromine, [Ar] 4s² 4p⁵, paints a picture of an atom with 35 electrons, two valence electrons, and a unique arrangement of electrons in its energy levels. Understanding the electron configuration of bromine is a fundamental step in unraveling the mysteries of its chemical behavior.
Molecular Orbital Theory: Unlocking the Secrets of Chemical Bonding
As we delve into the fascinating world of chemistry, understanding valence electrons is paramount. These electrons, residing in the outermost energy level of an atom, define its chemical reactivity. In this exploration, we’ll focus on the bromine molecule (Br2), unraveling the story of its chemical bond formation through the lens of Molecular Orbital Theory (MOT).
Formation of Molecular Orbitals:
Imagine the bromine atom with its seven valence electrons. MOT envisions these electrons combining to form molecular orbitals, which are regions around the atoms where the electrons are likely to be found. These orbitals arise from the overlap of atomic orbitals, the wave functions describing the electrons’ behavior in the individual atoms.
Types and Properties of Molecular Orbitals:
MOT classifies molecular orbitals into two primary types: bonding and antibonding orbitals. Bonding orbitals enhance the stability of the molecule by lowering its energy, while antibonding orbitals weaken the bond. Each molecular orbital possesses its own symmetry, influencing its shape and interaction with other orbitals.
Symmetry’s Influence on MOT:
The symmetry of the molecule plays a pivotal role in determining the types and properties of molecular orbitals. In the case of Br2, its linear shape results in symmetric molecular orbitals. The bonding orbitals are concentrated between the bromine nuclei, maximizing their overlap and strengthening the bond. The antibonding orbitals, in contrast, reside outside the internuclear region, minimizing overlap and destabilizing the molecule.
Through MOT, we gain a deeper understanding of chemical bonding. By examining the formation, types, and symmetry of molecular orbitals, we can explain the stability and reactivity of molecules like Br2. This powerful theory underpins numerous chemical applications, including the use of Br2 as a disinfectant, emphasizing its practical significance in our daily lives.
The Lewis Structure of Br2: Unraveling the Covalent Bond
In the realm of chemistry, the concept of valence electrons reigns supreme. These electrons, residing in the outermost energy level of an atom, determine the atom’s chemical behavior and its ability to form bonds with other atoms. Enter bromine, an element with seven valence electrons, poised to shed light on the fascinating world of covalent bonding.
The Br2 Molecule: A Tale of Two Bromines
Bromine, represented by the symbol Br, emerges as a diatomic molecule, meaning it exists as a pair of bromine atoms bound together. This union arises through the formation of a covalent bond, where the two atoms share their valence electrons.
Molecular Orbital Theory: A Quantum Leap
To delve deeper into the intricacies of Br2’s molecular bond, we enlist the aid of Molecular Orbital Theory (MOT). MOT posits that the atomic orbitals of the individual atoms overlap, giving rise to molecular orbitals that encompass the entire molecule. These molecular orbitals, with their distinct energies and shapes, play a crucial role in determining the molecule’s properties.
Representing Valence Electrons: The Lewis Structure
The Lewis structure, a graphical representation of an atom or molecule’s valence electrons, provides invaluable insights into Br2’s bonding. In the Lewis structure of Br2, each bromine atom contributes three valence electrons. These electrons form two pairs, each occupying a molecular orbital. The resulting covalent bond, symbolized by a dash between the two Br atoms (Br-Br), signifies the shared electrons.
Resonance: A Dynamic Duo
In Br2, resonance adds a layer of complexity to the bonding picture. Resonance suggests the existence of multiple equivalent Lewis structures that depict the same molecule. In the case of Br2, the presence of two equivalent Lewis structures indicates that the shared electrons are not confined to a single bond but rather resonate between the two bromine atoms. This resonance stabilizes the molecule, contributing to its overall bonding strength.
Bromine, with its seven valence electrons, provides a captivating case study for understanding covalent bonding. The Br2 molecule, held together by the sharing of valence electrons, exemplifies the power of MOT and the concept of resonance. By unraveling the mysteries of Br2’s Lewis structure, we gain a deeper appreciation for the intricate dance of valence electrons and the formation of chemical bonds that shape the world around us.