Bromine Chemistry: Delving Into Valence Electrons And Electronic Configuration

Bromine (Br), a halogen, possesses 7 valence electrons in its outermost shell, represented by the electronic configuration [Ar] 4s² 3d¹⁰ 4p⁵. Valence electrons dictate an element’s chemical behavior, and bromine’s high reactivity stems from its electronegativity and tendency to gain electrons, forming halide ions. This shared characteristic among halogens, all with 7 valence electrons, contributes to their similar chemical properties. Understanding the interplay between valence electrons and electronic configuration provides valuable insights into bromine’s reactivity and bonding behavior.
Valence Electrons: Unlocking the Secrets of Bromine’s Chemistry
In the realm of chemistry, bromine (Br) is an enigmatic element that holds both intrigue and importance. Halogens, a peculiar family of elements, it’s here where we find bromine residing. Its chemical properties, unique in their own right, stem from the very foundation of its atomic structure, particularly its valence electrons. These electrons, like tiny messengers, dictate the element’s behavior and open the door to understanding its chemistry.
The Significance of Valence Electrons: Shaping Chemical Destiny
Valence electrons are those outermost electrons in an atom’s orbit, the ones that determine how the atom interacts with its surroundings. They hold the key to understanding an element’s chemical behavior, as they are the gateways through which the atom forms bonds with others.
Bromine’s Electronic Dance: A Symphony of Seven
Bromine, with its atomic number of 35, possesses seven valence electrons. Its electronic configuration, [Ar] 4s² 3d¹⁰ 4p⁵, is a molecular fingerprint that reveals the distribution of these electrons. The seven valence electrons, residing in the outermost shell, are the architects of bromine’s chemical prowess.
Halogens: United by Valence
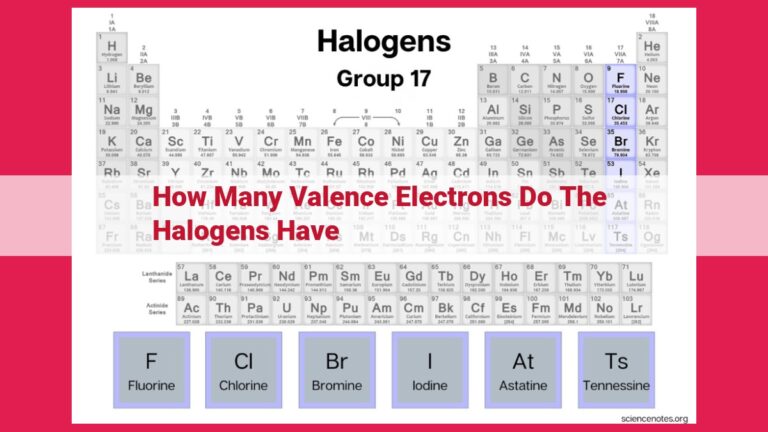
All halogens—fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At)—share a common bond: they all possess seven valence electrons. This commonality bestows upon them a remarkable similarity in chemical properties, making them highly reactive and eager to form strong bonds.
Electrons Unleashed: The Reactivity of Bromine
Bromine’s seven valence electrons are like eager dancers, constantly seeking partners to form stable bonds. This high chemical reactivity is a hallmark of elements with seven valence electrons. Their strong electronegativity, a measure of their attraction for electrons, drives them to pull electrons from other atoms, forming negatively charged ions called halide ions.
Bromine’s Role in the Chemical Landscape
The unique properties of bromine, dictated by its valence electrons, make it an indispensable player in the chemical world. It finds applications in a wide range of industries, from water purification to photography to medicine. Understanding its valence electrons is crucial for unlocking its full potential in various chemical reactions and technological advancements.
The Significance of Valence Electrons in Predicting Chemical Behavior
In the fascinating world of chemistry, understanding the fundamental principles that govern the behavior of elements is crucial for unraveling their mysteries. Among these principles, the concept of valence electrons stands out as a key determinant of an element’s chemical properties.
Imagine atoms as tiny universes, with electrons orbiting their nuclei like celestial bodies. Valence electrons are the outermost electrons, located in the outermost energy level of an atom. These electrons play a pivotal role in determining how an element interacts with others, shaping its chemical behavior and reactivity.
The number and arrangement of valence electrons provide vital clues about an element’s:
-
Electronegativity: Valence electrons determine how strongly an atom attracts electrons from other atoms, forming chemical bonds. Elements with a high number of valence electrons tend to be more electronegative, meaning they have a stronger pull on electrons.
-
Reactivity: Valence electrons govern an element’s tendency to participate in chemical reactions. Elements with a small number of valence electrons (1-3) are generally less reactive, while those with many valence electrons (4-7) are highly reactive.
By understanding the concept of valence electrons, chemists can make educated predictions about an element’s behavior, paving the way for countless discoveries and innovations in the field of chemistry.
Unlocking the Secrets of Bromine’s Valence Electrons
In the realm of chemistry, understanding the intricate dance of electrons is crucial. One such element, bromine, plays a captivating role due to its unique electronic configuration. Let us delve into the fascinating world of bromine’s valence electrons and unravel the profound impact they have on its chemical behavior.
Counting the Guardians: Valence Electrons in Bromine
Bromine, with its atomic number 35, belongs to the halogen family. These elements share a common trait: they possess seven valence electrons, which act as guardians of their chemical reactivity. Valence electrons determine an element’s eagerness to form bonds and interact with other atoms.
Unveiling the Electronic Fingerprint: Bromine’s Configuration
Every atom carries a unique fingerprint known as its electronic configuration. Bromine’s electronic configuration, written as [Ar] 4s² 3d¹⁰ 4p⁵, reveals the arrangement of its electrons in different energy levels or shells. The outermost shell, known as the valence shell, houses the seven valence electrons, represented as 4p⁵.
The Halide Connection: Valence Electrons and Chemical Reactivity
The number of valence electrons plays a pivotal role in shaping an element’s chemical behavior. Elements with seven valence electrons, like halogens, possess an insatiable desire to acquire more electrons to complete their valence shells. This insatiable quest makes halogens highly reactive and electronegative, meaning they have a strong tendency to attract electrons from other atoms.
Halogens in Harmony: A Shared Destiny
All halogens (fluorine, chlorine, bromine, iodine, and astatine) share the common thread of having seven valence electrons. This shared electronic configuration bestows upon them similar chemical properties, making them eager partners in forming bonds and influencing the chemistry of the world around us.
The exploration of bromine’s valence electrons has illuminated its path in the captivating world of chemistry. Understanding the number and arrangement of these electrons provides a framework for comprehending bromine’s reactivity, bonding behavior, and overall chemical fingerprint. Valence electrons serve as guiding stars, illuminating the intricate dance of atoms and shaping the molecular tapestry of our universe.
Properties of Elements with 7 Valence Electrons
Halogens: The Elements of Intrigue
In the captivating world of chemistry, elements with seven valence electrons hold a special place. These elements, known as halogens, boast an irresistible charm that has captivated scientists for centuries. Their exceptional characteristics stem from their high chemical reactivity, making them essential players in countless chemical reactions.
Electronegativity: The Driving Force of Reactivity
The key to understanding the reactivity of halogens lies in their electronegativity. This property quantifies an atom’s ability to attract electrons towards itself. Halogens possess exceptionally high electronegativity, giving them an insatiable appetite for electrons. This hunger drives their tendency to form chemical bonds, eager to complete their outer electron shell and achieve stability.
Halide Ions: The Stable Outcome
With their voracious appetite for electrons, halogens often engage in chemical reactions where they attract electrons from other atoms. This process leads to the formation of negatively charged ions known as halide ions. For instance, the element bromine readily accepts an electron to form the bromide ion (Br-). This transformation highlights the halogens’ penchant for gaining electrons to achieve electronic stability.
In conclusion, elements with seven valence electrons, such as halogens, exhibit exceptional chemical reactivity due to their high electronegativity. Their relentless pursuit of electrons drives their tendency to form chemical bonds, resulting in the formation of halide ions and unlocking a plethora of fascinating chemical possibilities.
Halogens: The Guardians of Seven Valence Electrons
In the realm of chemistry, the halogens stand out as a fascinating group of elements that share a remarkable trait: they each possess seven valence electrons. These valence electrons, the outermost electrons in an atom’s electronic structure, play a pivotal role in determining an element’s chemical behavior and the dance it engages in with other substances.
The halogens, a tight-knit family comprising fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At), share this electronic fingerprint. This shared electronic configuration endows them with a common set of chemical traits, making them reactive partners in the world of chemistry.
The seven valence electrons that these elements flaunt grant them a strong electronegativity, a measure of their ability to attract electrons from other atoms. This insatiable craving for electrons drives their chemical reactivity, making them eager to form halide ions by capturing an additional electron.
Fluorine, the most electronegative of the halogens, is an aggressive electron-grabber, swiftly forming fluoride ions. Chlorine, a close second, is a renowned disinfectant, eliminating bacteria and viruses by stealing electrons from their essential proteins. Bromine, with its distinctive reddish-brown hue, finds its niche in chemistry as a versatile reagent, engaging in a wide range of reactions.
Iodine, a solid with a lustrous sheen, boasts a unique ability to sublime, transforming directly from a solid to a gas without passing through the liquid phase. Astatine, the radioactive heavyweight of the halogen family, is a rare and elusive element with a fascinating story to tell.
Each halogen, with its unique personality shaped by its seven valence electrons, plays a vital role in the symphony of chemistry.
Unveiling the Secrets of Bromine’s Chemistry: The Interplay of Valence Electrons and Electronic Configuration
The tale of bromine’s reactivity begins with its valence electrons. These crucial electrons, perched on the outermost energy level of the atom, are the gatekeepers of chemical interactions. Bromine boasts 7 valence electrons, an attribute that paints a vibrant picture of its chemical behavior.
This electronic configuration, [Ar] 4s² 3d¹⁰ 4p⁵, is a blueprint that governs bromine’s chemical nature. The stable noble gas core (Ar) shields the valence electrons from the nucleus’s pull, making them eager to participate in chemical bonding.
The synergy between the **7 valence electrons and the electronic configuration orchestrates bromine’s exceptional reactivity.** This combination endows halogens, including bromine, with a strong electronegativity. This insatiable desire to attract electrons propels bromine into forming negative ions (halide ions), which play a pivotal role in countless chemical reactions.
The prevalence of **7 valence electrons across the halogen family (F, Cl, Br, I, At) paints a cohesive picture of their shared chemical traits.** This electronic kinship manifests in their high reactivity, tendency to form halide ions, and similar physical properties.
In conclusion, bromine’s chemistry is a captivating dance orchestrated by the interplay of its **7 valence electrons and electronic configuration. This intricate choreography underlies bromine’s reactivity and bonding behavior, illuminating the essence of its chemical nature.**