Boron’s Valence Electrons: Key To Chemical Behavior And Technological Applications
Boron, a metalloid element with atomic number 5, possesses three valence electrons distributed in its 2p orbital. These valence electrons dictate its chemical behavior as a Lewis acid and determine its position in Group 13 of the periodic table. Boron’s valence electrons play a pivotal role in molecular bonding, influencing its reactivity and applications in materials science and catalysis. Understanding the significance of valence electrons is crucial for unraveling the diverse chemical properties and technological implications of boron.
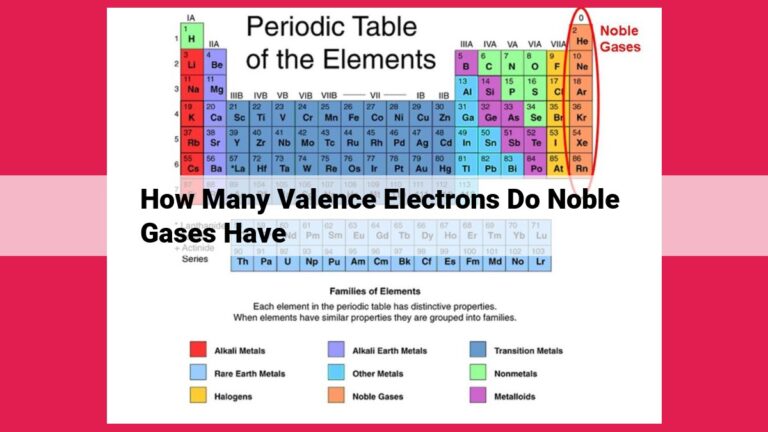
Valence Electrons: The Cornerstone of Chemistry
In the realm of chemistry, electrons play a pivotal role in shaping the behavior and interactions of atoms. Among these electrons, a special group known as valence electrons holds immense significance. These outermost electrons, located in a specific energy level of an atom, determine its chemical properties and bonding capabilities.
Valence electrons are the key players in the formation of chemical bonds, the forces that hold atoms together to form molecules and compounds. By sharing or exchanging valence electrons, atoms can achieve a stable electron configuration, resulting in the creation of a vast array of chemical substances.
The number of valence electrons an atom possesses directly influences its chemical reactivity. This number is directly related to the atomic number of the element, which identifies the number of protons in the nucleus and, consequently, the number of electrons in the atom.
Understanding Boron: A Curious Element with Three Valence Electrons
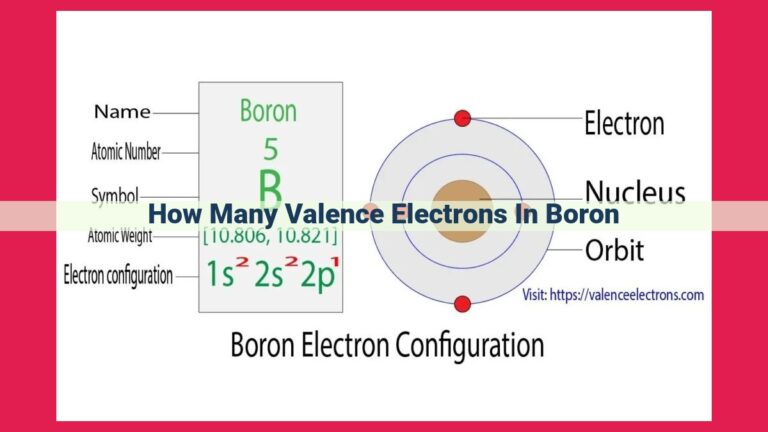
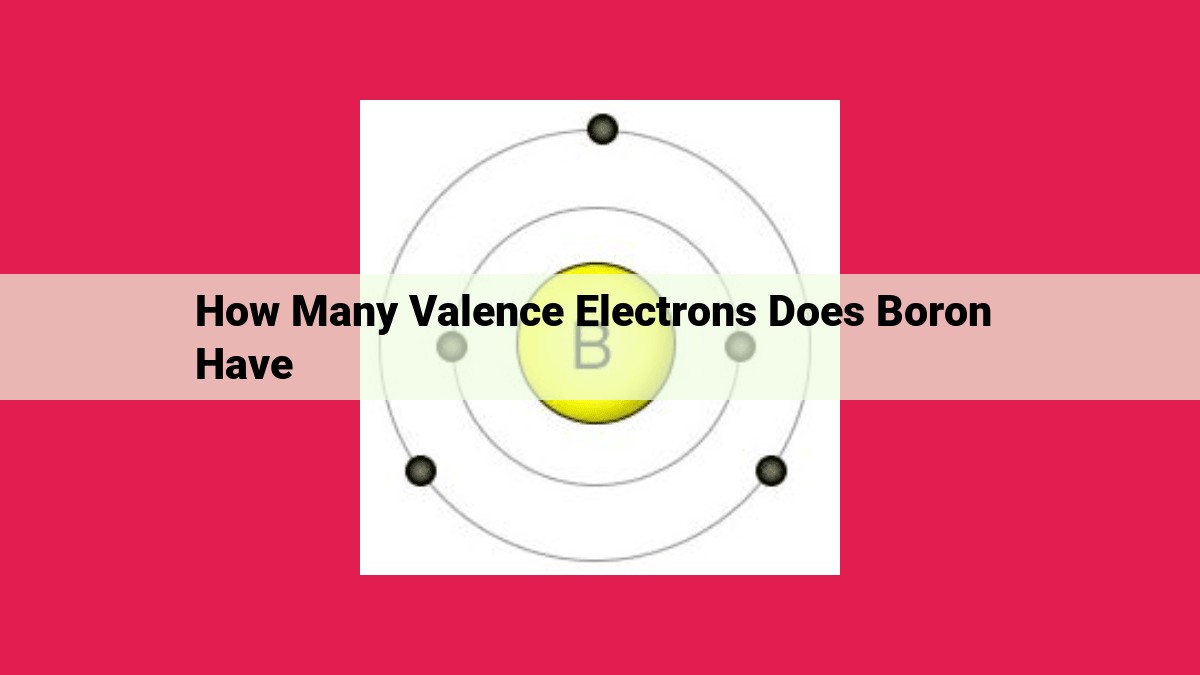
Boron, an enigmatic element classified as a metalloid, resides in Group 13 of the periodic table. With an atomic number of 5, boron’s electron configuration can be represented as 2s2 2p1. This configuration reveals that boron has three valence electrons in its outermost 2p orbital.
The presence of three valence electrons in boron profoundly impacts its chemical behavior. As a Lewis acid, boron readily accepts electron pairs from other atoms or molecules to complete its octet of valence electrons, achieving a more stable configuration. This characteristic enables boron to form covalent bonds and participate in various chemical reactions.
Boron’s unique chemical properties, largely influenced by its three valence electrons, have led to its widespread applications in materials science and catalysis. By understanding the fundamental role of valence electrons in boron’s chemistry, scientists and engineers can harness its potential for groundbreaking innovations and advancements.
How Many Valence Electrons Does Boron Have?
Valence electrons are the outermost electrons in an atom’s electron cloud. They play a crucial role in chemical bonding and determine the reactivity of an element.
Boron: A Tale of Metalloid Mystery
Boron’s Chemical Identity
Boron is a captivating element from Group 13 of the periodic table. Uniquely classified as a metalloid, it exhibits properties that bridge the gap between metals and non-metals.
The Chemical Chameleon: A Lewis Acid
Boron’s chemical behavior is that of a Lewis acid. It readily accepts electrons from other atoms or molecules, forming covalent bonds. This characteristic makes it an essential player in various chemical reactions.
Atomic Number and Electron Configuration
Atomic Number and Elemental Identity
The atomic number of an element defines its unique identity on the periodic table. Boron, with an atomic number of 5, contains five protons in its nucleus.
Electron Configuration: A Blueprint for Electrons
Each element has a distinct arrangement of electrons in shells around its nucleus. This arrangement, known as electron configuration, reveals the number of valence electrons.
Boron’s Electron Configuration
Boron’s electron configuration is 2s2 2p1. This arrangement signifies that it has two valence electrons in the 2p orbital.
Valence Electrons in Boron
Three Valence Electrons: A Chemical Conundrum
Boron is characterized by three valence electrons that reside in its outermost shell. These electrons dictate its chemical bonding capabilities and reactivity.
Chemical Bonding and Group 13
Boron’s three valence electrons place it in Group 13 of the periodic table. This positioning highlights boron’s ability to form three covalent bonds, making it a versatile building block in various chemical compounds.
Unveiling Boron’s Valency: A Journey to the Heart of the Atom
In the realm of chemistry, valence electrons play a pivotal role in shaping the behavior of elements. These are the electrons that occupy the outermost energy level of an atom, eager to participate in chemical reactions and form molecular bonds. Join us on an engaging journey as we delve into the intriguing case of boron, an element that captivates scientists with its distinctive metalloid character.
Atomic Number and Electron Configuration: The Keys to Elemental Identity
Every atom’s individuality is defined by its atomic number, a unique number representing the quantity of protons within its nucleus. For boron, this number stands at 5, revealing its special status as the fifth element in the periodic table.
Beyond the nucleus, electrons dance in intricate patterns around the atom’s core. This arrangement, known as electron configuration, follows precise rules, with the outermost electrons residing in the energy level with the highest number. In boron’s case, its electron configuration is 2s2 2p1, indicating two electrons in the 2s subshell and one lone electron in the 2p subshell.
Unraveling Boron’s Valency: The Power of Three
The number of valence electrons in an atom holds great significance for its chemical reactivity. Boron possesses three valence electrons, residing in the 2p subshell. These three electrons are keen to interact with other atoms, either by sharing or gaining electrons, thereby forming chemical bonds.
Boron’s position in Group 13 of the periodic table is a direct consequence of its three valence electrons. This placement alongside other elements with the same valence electron count underscores the profound influence of this atomic characteristic on chemical behavior.
Applications and Significance: Boron’s Chemical Footprint
The understanding of boron’s valence electrons is essential for unraveling its remarkable chemical properties. Its ability to form strong bonds with various elements has made it an indispensable component in diverse fields.
In materials science, boron’s incorporation into materials like ceramics and semiconductors enhances their strength, durability, and electronic properties. Additionally, its catalytic prowess makes it indispensable in industrial processes, where it facilitates reactions with precision and efficiency.
Our exploration of boron’s valence electrons has provided a glimpse into the captivating world of atomic structure and chemical bonding. By weaving together scientific concepts and storytelling elements, we hope to foster a deeper appreciation for the intricacies of chemistry. This blog post serves as a testament to the power of science to engage and enlighten, inviting you on a continuing journey of exploration and discovery.
Valence Electrons in Boron: Unraveling the Chemical Enigmas
In the realm of chemistry, the concept of valence electrons holds immense significance. Boron, a fascinating element positioned in Group 13 of the periodic table, exemplifies the profound influence of valence electrons on an element’s chemical characteristics.
Boron’s Electronic Composition
Boron bears an atomic number of 5, which implies that its nucleus harbors 5 positively charged protons. In its electronic configuration, the electrons are arranged as follows:
- 1s²: Two electrons occupy the innermost energy level.
- 2s²: The second energy level accommodates two more electrons.
- **2p¹: The third energy level contains a lone electron, which is crucial for understanding boron’s chemical behavior.*
The Power of Three Valence Electrons
The three valence electrons that reside in boron’s outermost energy level play a pivotal role in shaping its chemical properties. These electrons are loosely bound to the atomic nucleus, making them highly reactive and eager to participate in chemical bonding.
Bridging the Bonding Divide
The presence of three valence electrons places boron in Group 13 of the periodic table, alongside other elements with similar electronic configurations. This grouping highlights boron’s capacity to form stable compounds by sharing or donating electrons.
Chemical Bonding Capabilities
Boron’s three valence electrons enable it to engage in a variety of chemical bonding interactions. It can form covalent bonds by sharing electron pairs with other atoms, creating molecules such as boron trifluoride (BF3) and boric acid (H3BO3). Additionally, boron can act as a Lewis acid, accepting electron pairs from other atoms to form coordinate covalent bonds.
Industrial Significance
The unique chemical behavior of boron, largely influenced by its valence electrons, has garnered significant industrial applications. For instance, boron-based materials find widespread use in the production of semiconductors, glass, and ceramics. Additionally, boron compounds serve as catalysts in various chemical reactions, enhancing their efficiency and reducing energy consumption.
Delving into the world of boron’s valence electrons unveils an intricate tapestry of chemical characteristics that governs its behavior and paves the way for its myriad applications. By understanding the profound impact of these electrons, we gain valuable insights into the complexities of chemical bonding and the remarkable versatility of boron as a chemical element.
How Many Valence Electrons Does Boron Have?
Valence Electrons: A Chemical Bonding Primer
In the realm of chemistry, understanding valence electrons is crucial for deciphering the behavior and bonding tendencies of elements. These electrons, residing in an atom’s outermost shell, play a pivotal role in determining its chemical interactions.
Boron: A Metalloid with a Unique Valence
Boron, a metalloid positioned in Group 13 of the periodic table, presents a fascinating case study. Its unique chemical behavior stems from its three valence electrons. This distinctive number not only defines boron’s position within the periodic table but also shapes its bonding versatility.
Applications of Boron’s Chemical Properties
Harnessing the power of boron’s valence electrons has led to groundbreaking applications in diverse fields. In materials science, boron-containing compounds find use in aerospace composites, high-temperature materials, and semiconductors. Its unique reactivity has also positioned boron as a key player in catalysis, where it aids in chemical reactions by enhancing catalytic activity.
As we delve into the intricate world of chemistry, encompassing concepts like valence electrons and their profound impact on elemental behavior, we gain a deeper appreciation for the building blocks of our universe. Boron, with its distinctive valence electron configuration, serves as a testament to the captivating power of chemistry and its ability to shape the world around us.