Boron: An Essential Element At Atomic Number 5
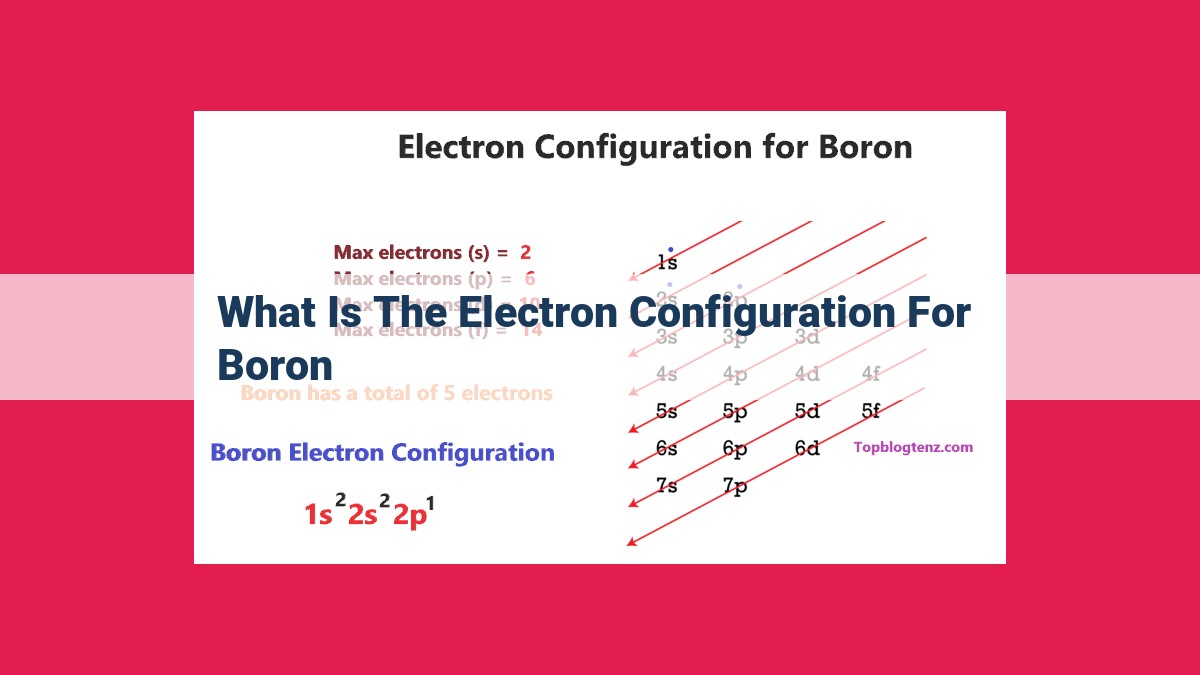
Boron, with an atomic number of 5, has an electron configuration of 1s²2s²2p¹. The electrons are distributed in three energy levels: two in the 1s orbital, two in the 2s orbital, and one in the 2p orbital. The single electron in the 2p orbital is the valence electron, responsible for boron’s chemical properties and bonding behavior.
Understanding Atomic Number
- Define atomic number and its relation to the number of protons.
- Explain its significance in determining the element and its position on the periodic table.
Understanding the Atomic Number: A Journey into the Heart of Matter
Every atom, the fundamental building block of matter, possesses a unique identity determined by its atomic number. This number, a crucial piece of the atomic puzzle, reveals the very essence of an element and its place within the vast tapestry of the universe.
The atomic number is synonymous with the number of protons that reside within the atom’s nucleus, the dense core at its heart. Protons, positively charged particles, are the unwavering foundation upon which atoms are built. The atomic number, therefore, is a direct gateway to the atom’s elemental identity.
Each element in the periodic table, from the familiar hydrogen to the enigmatic uranium, has its own unique atomic number. This number determines the element’s place in the table, arranging them in an orderly fashion that reflects their shared properties and behaviors. The atomic number, like a celestial compass, guides us in navigating the enigmatic realm of the elements.
Electron Configuration: Unveiling the Symphony of Electrons
In the captivating realm of chemistry, understanding the electron configuration of atoms is paramount. It unveils the intricate arrangement of electrons within atoms, dictating their behavior and properties. This knowledge forms the foundation for comprehending the vast tapestry of chemical reactions.
Energy Levels: The Stairway to Electron Stability
Electrons reside in specific energy levels within an atom, akin to dancers performing on a tiered stage. These levels are arranged like steps, with each level containing a fixed number of electrons. The lowest energy level, aptly named the ground state, provides the most stable haven for electrons.
Orbitals: The Electrons’ Dancing Spaces
Within each energy level, electrons occupy designated spaces called orbitals, akin to the positions dancers take on a stage. Orbitals are described by their shape and orientation, creating a complex three-dimensional framework. The first energy level houses only one orbital, the “s” orbital, which is spherical in shape. Subsequent energy levels introduce additional orbitals, such as the “p,” “d,” and “f” orbitals, with more intricate shapes.
Electron Distribution: A Matter of Atomic Number
The atomic number of an element, the cornerstone of its identity, governs the number of electrons it possesses. Electrons are distributed among the available energy levels and orbitals in a systematic manner. The lowest energy levels are filled first, followed by the higher ones. For instance, hydrogen, with one electron, occupies the 1s orbital. Helium, with two electrons, fills both the 1s orbitals.
Delving into the realm of electron configuration equips us with a profound understanding of the intricate architecture of atoms. By unraveling the energy levels, orbitals, and electron distribution, we gain a glimpse into the fundamental principles that govern the behavior of matter. This knowledge serves as a sturdy bridge, connecting the microscopic world of atoms to the macroscopic world of the elements and the chemical reactions that shape our universe.
Valence Electrons: Key Players in Chemical Reactions
In the realm of chemistry, valence electrons are like the social butterflies of the atomic world, playing a crucial role in determining an element’s personality and its ability to form bonds with other atoms.
These special electrons reside in the outermost energy level of an atom, like the outermost ring of seats in a theater. They are the most energetic and most likely to interact with the outside world, making them vital for chemical reactions.
The number of valence electrons an element possesses is a major factor in determining its chemical properties. For instance, atoms with a full set of valence electrons, like helium, are highly stable and inert. They don’t readily react with other elements because they have no need for more electrons.
On the other hand, atoms with incomplete valence electron shells, like sodium and chlorine, are much more reactive. They are eager to gain or lose electrons to achieve a stable configuration. This drive to complete their valence shells is what fuels chemical reactions.
Valence electrons are like the matchmakers of the atomic world. They mediate the formation of chemical bonds, the forces that hold atoms together to form molecules. When atoms have unpaired valence electrons, they can share or transfer these electrons with other atoms to create stable bonds.
For example, sodium has one valence electron, which it readily gives up to other atoms. Chlorine, on the other hand, has seven valence electrons and needs one more to complete its shell. When these two atoms meet, sodium donates its valence electron to chlorine, forming the stable sodium chloride molecule.
Understanding valence electrons is essential for understanding chemical bonding and predicting the reactivity of elements. These tiny particles are the driving force behind the formation of molecules and the countless chemical reactions that shape our world.
Orbital Diagrams: Unveiling the Invisible Architecture of Atoms
Introduction:
Embark on a captivating journey into the fascinating realm of atoms, where we’ll uncover the secrets of orbital diagrams. These diagrams are not mere abstract representations but rather powerful tools that reveal the intricate arrangement of electrons within atoms, providing us with invaluable insights into their behavior and properties.
Understanding Orbital Diagrams:
Think of an orbital diagram as a snapshot of an atom’s electron configuration. It depicts the energy levels and orbitals, which are like the celestial bodies orbiting a star. Each orbital can accommodate a maximum of two electrons, and their arrangement determines many of an atom’s fundamental characteristics.
Predicting Atomic Structure and Molecular Symmetry:
Orbital diagrams aren’t just decorative; they hold immense predictive power. By studying the arrangement of electrons in an atom, we can anticipate the atom’s shape, magnetism, and even the types of chemical bonds it can form. For molecules, orbital diagrams allow us to understand their symmetry, a crucial factor in determining their chemical reactivity and physical properties.
Unveiling the Secrets of Ground State:
Every atom has a preferred arrangement of electrons known as the ground state, where its energy is at its minimum. In the ground state, electrons occupy the most stable orbitals, resembling a perfectly balanced dance. Understanding the ground state is essential for comprehending the reactivity and stability of atoms.
Conclusion:
Orbital diagrams are not just complex representations; they are windows into the very heart of matter. Through these diagrams, we can peer into the unseen world of atoms, gaining unprecedented knowledge of their structure, behavior, and the fascinating world they orchestrate around us.
Ground State: The Key to Understanding Atomic Stability
Every atom exists in a state of equilibrium, a delicate balance between its energy levels. The ground state represents the atom’s most stable configuration, where its electrons reside in the lowest energy orbitals available.
In the ground state, electrons occupy orbitals in an orderly fashion, beginning with the lowest energy levels and working their way up. These electrons are arranged in a way that minimizes the atom’s overall energy, creating a stable and balanced system.
In contrast to the ground state, excited states occur when electrons are temporarily promoted to higher energy orbitals. This can happen when an atom absorbs energy, such as from light or heat. Excited states are unstable, and electrons quickly return to the ground state, releasing the excess energy as photons.
Understanding the ground state is crucial for predicting the behavior of atoms. It influences:
- Atomic Structure: The arrangement of electrons in the ground state determines the atom’s size, shape, and reactivity.
- Chemical Properties: Ground state electrons participate in chemical reactions, determining an element’s ability to form bonds and interact with other atoms.
- Electronic Transitions: Transitions between ground and excited states are responsible for phenomena such as light absorption, emission, and energy transfer.