Unlocking The Secrets Of Atomic Structure: Bohr’s Revolutionary Refinement Of Rutherford’s Model
Bohr’s atomic model differed significantly from Rutherford’s. Bohr introduced quantized energy levels and fixed circular orbits for electrons, explaining atomic spectra and electron stability. Rutherford’s model, with electrons orbiting in a diffuse cloud, lacked these features. Bohr’s model refined Rutherford’s by addressing key limitations and providing a deeper understanding of atomic structure and the behavior of electrons.
The Evolution of Atomic Models: A Tale of Bohr and Rutherford
Before we delve into the fascinating world of atomic models, let’s set the stage. Niels Bohr and Ernest Rutherford, two brilliant scientific minds, played pivotal roles in shaping our understanding of the atom. Bohr’s model revolutionized atomic physics, while Rutherford’s groundbreaking experiments paved the way for further discoveries.
Bohr’s Atomic Model: A Quantum Revolution
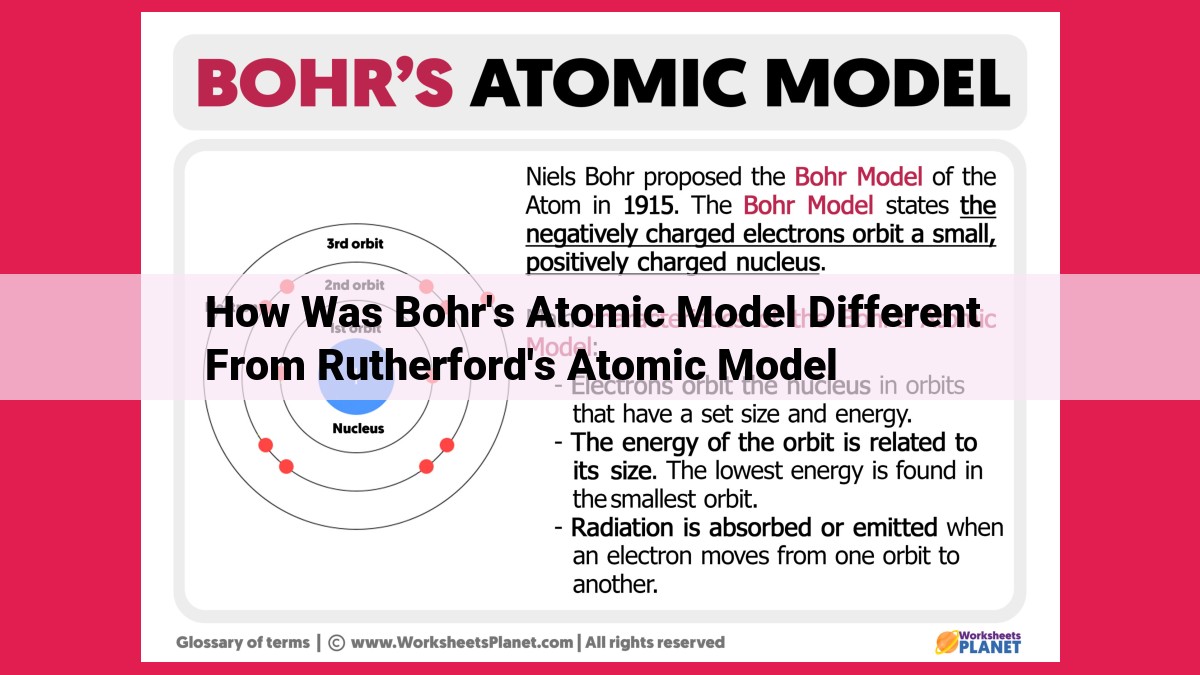
In 1913, Niels Bohr proposed a groundbreaking model of the atom. Key features included:
- Quantization: Electrons occupy discrete energy levels, like steps on a ladder.
- Fixed Orbits: Electrons orbit the nucleus in specific, fixed paths defined by quantum numbers.
- Nuclear Center: A small, positively charged nucleus is the atom’s core.
Bohr’s model brilliantly explained the unique emission and absorption spectra of hydrogen atoms, a phenomenon that had puzzled scientists for years. This quantum approach revolutionized physics and provided a solid foundation for understanding atomic behavior.
Rutherford’s Atomic Model: A Glimpse into the Atom’s Heart
Ernest Rutherford’s groundbreaking experiments in 1911 shattered the prevailing “plum pudding model” of the atom. His model hinted at the atom’s intricate structure:
- Diffuse Cloud: Electrons float in an amorphous cloud, not bound to fixed orbits.
- Unquantized Orbits: Electron paths are undefined and non-circular.
- Tiny Nucleus: A dense, positively charged nucleus, much smaller than the atom itself.
Rutherford’s model, though less precise than Bohr’s, laid the groundwork for further advancements in atomic theory. It revealed the atom’s vast, empty space and hinted at the presence of a force that held the nucleus together.
Bohr’s Revolutionary Atomic Model: A Tale of Quantization and Energy Levels
In the early 1900s, Niels Bohr made a groundbreaking contribution to our understanding of the atom. His atomic model revolutionized the field of physics and laid the groundwork for modern quantum theory.
Key Features of Bohr’s Atomic Model
Bohr’s model was based on the following key features:
- Quantized Energy Levels: Electrons in an atom are restricted to orbiting the nucleus at specific, quantized energy levels. These levels are analogous to the steps of a ladder, with each step representing a different energy level.
- Circular Orbits: Electrons orbit the nucleus in circular paths of specific radii. These orbits are determined by the electron’s energy level. The lower the energy level, the closer the orbit is to the nucleus.
- Quantum Numbers: Each orbit is uniquely defined by three quantum numbers: the principal quantum number (n), the angular momentum quantum number (l), and the magnetic quantum number (ml). These numbers specify the electron’s energy, shape of its orbit, and orientation in space.
- Small, Positively Charged Nucleus: The atom’s nucleus is a small, dense, positively charged region located at the center. Protons, which have a positive charge, reside in the nucleus.
The Significance of Bohr’s Model
Bohr’s model was a major advancement in atomic physics because it explained several previously puzzling phenomena, including:
- Emission and Absorption of Light: Bohr’s model predicted that electrons can transition between energy levels by absorbing or emitting photons of light. This explained the observed emission and absorption spectra of atoms.
- Atomic Stability: Bohr’s model explained why atoms are stable and do not collapse. Electrons in the lowest energy level are stable because they have no lower energy state to transition to.
- Periodic Table: Bohr’s model helped to explain the periodic properties of elements by showing how the arrangement of electrons in energy levels determines an element’s chemical behavior.
Bohr’s model was a significant milestone in the history of physics. It provided the first comprehensive description of the atom and laid the foundation for future developments in quantum theory.
Rutherford’s Revolutionary Atomic Model (1911)
The Quest for Unraveling the Atomic Enigma:
Before 1911, the nature of atoms remained shrouded in mystery. Scientists grappled with the challenge of understanding the tiny particles that composed all matter. It was Ernest Rutherford, a renowned physicist from New Zealand, who would forever alter our perception of the atomic realm with his groundbreaking model.
Rutherford’s Experiment and a Serendipitous Discovery:
Rutherford’s atomic model emerged from a series of brilliant experiments conducted in 1911. In his famous gold foil experiment, he bombarded a thin sheet of gold foil with alpha particles, positively charged helium nuclei. Astonishingly, most of the alpha particles passed through the foil as if it were nearly empty space. However, a small percentage of the particles were deflected at large angles, indicating the presence of a tiny, dense nucleus at the heart of the atom.
Unveiling the Structure of the Atom:
Rutherford’s experiment revealed a completely new picture of the atom. He proposed a model that had the following characteristics:
- Dense Nucleus: The nucleus, located at the center of the atom, was extremely small and contained positively charged protons.
- Diffuse Electron Cloud: Surrounding the nucleus was a vast, diffuse cloud of electrons.
- Unquantized Orbits: Unlike Bohr’s model, Rutherford’s electrons did not orbit the nucleus in fixed, circular paths. Instead, they moved in unquantized, elliptical orbits.
Limitations of Rutherford’s Model:
While Rutherford’s model was a major breakthrough, it also had its limitations. It failed to explain why electrons did not spiral into the nucleus and why certain atoms emitted specific wavelengths of light. These questions would remain unanswered until Niels Bohr introduced his revolutionary model a few years later.
Historical Significance:
Rutherford’s atomic model marked a paradigm shift in our understanding of atoms. It laid the foundation for modern physics and paved the way for the development of Bohr’s quantized model, which would further refine our understanding of atomic structure.
Key Differences between Bohr’s and Rutherford’s Atomic Models
Electrons’ Energy:
In Bohr’s model, electrons reside in quantized energy levels, meaning they can only occupy specific, defined orbits. This contrasts with Rutherford’s model, where electrons move in a diffuse cloud with continuous energy distribution.
Orbits:
Under Bohr’s model, electrons orbit the nucleus in fixed, circular paths of specific radii. These orbits are determined by quantum numbers. In contrast, Rutherford’s model depicts electrons moving in unfixed, elliptical paths with varying radii.
Stability:
Bohr’s model introduced the concept of energy levels to explain electron stability. Electrons in lower energy levels are more stable, while those in higher energy levels are unstable_ and can transition to lower levels by emitting energy as light. Rutherford’s model, on the other hand, did not provide an explanation for electron stability.
Radiation:
Bohr’s model explains atomic spectra as a result of electron transitions between energy levels. When an electron absorbs energy_, it moves to a higher energy level, and when it releases energy_, it transitions to a lower level, emitting photons of light of specific frequencies. Rutherford’s model, lacking the concept of energy levels, could not explain atomic spectra.