Biological Buffers: Essential Regulators Of Ph In Living Organisms For Seo
Biological buffers are solutions that resist changes in pH, crucial for maintaining pH homeostasis in living organisms. These buffers consist of a weak acid and its conjugate base or a weak base and its conjugate acid, where the pKa value determines their effectiveness. Buffers neutralize added acids or bases by converting them to weaker acids or acids, respectively. The three main types of biological buffers are the carbonic acid/bicarbonate buffer, phosphate buffer, and protein buffer. Buffer capacity, influenced by concentration and pKa, measures their ability to resist pH changes.
- Explain the concept of buffers as solutions that resist pH changes
- Highlight the importance of biological buffers in maintaining pH homeostasis in living organisms
Biological Buffers: The Sentinels of pH Stability
In the intricate tapestry of life, maintaining a stable pH environment is paramount. Enter biological buffers, the unsung heroes that vigilantly guard against pH fluctuations, ensuring the harmonious functioning of cells and organisms.
Defining Biological Buffers
Buffers are clever solutions that steadfastly resist changes in pH. They comprise two key components: a weak acid and its conjugate base, or a weak base and its conjugate acid. Imagine a pH tug-of-war, where buffers play the role of peacemakers, deftly neutralizing both excess acids and bases, maintaining the delicate pH balance.
The Significance of Biological Buffers
Biological buffers are indispensable for life. pH homeostasis is crucial for countless biochemical reactions within cells and tissues. Any significant pH deviation can disrupt enzyme activity, alter protein structure, and even compromise cell viability. In living organisms, biological buffers act as tireless guardians, ensuring that pH remains within a narrow and life-sustaining range.
Components of Biological Buffers
Understanding the Essence of Buffers
In the realm of chemistry, biological buffers stand out as extraordinary solutions that possess the unique ability to resist changes in pH (acidity or basicity). Their presence is crucial for maintaining the delicate pH homeostasis in living organisms, ensuring the proper functioning of cells and organs.
Types of Buffer Components
Biological buffers are composed of two distinct components:
-
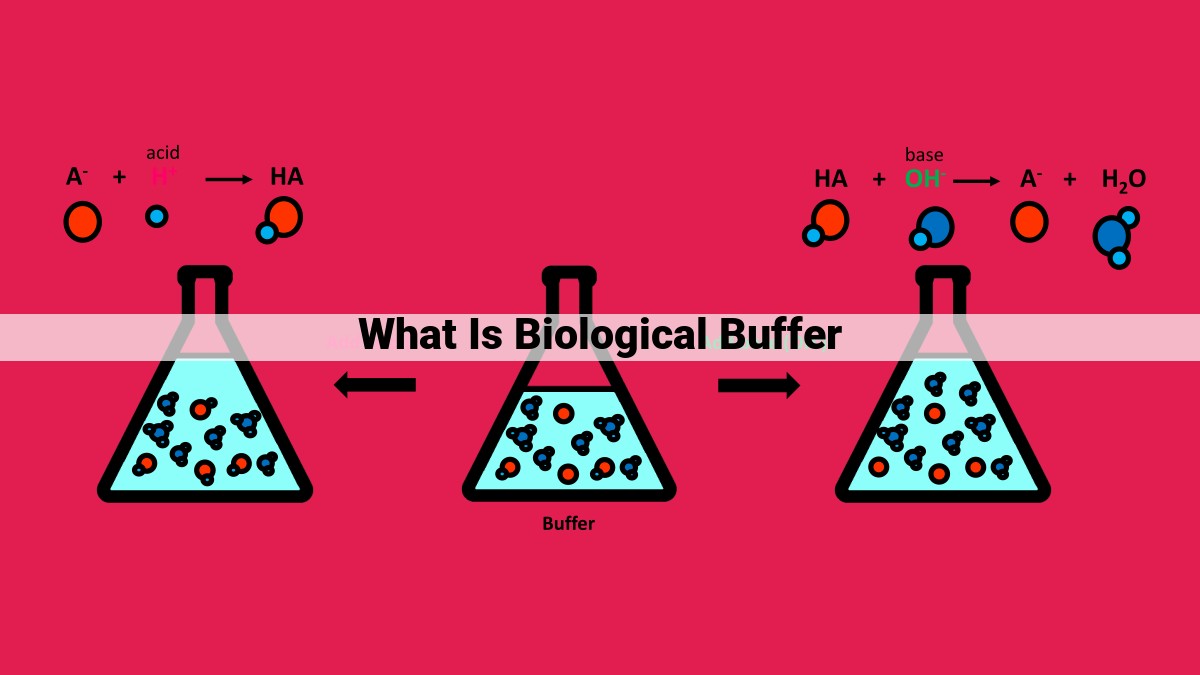
Weak Acid and Conjugate Base: These buffers consist of a weak acid (HA) and its conjugate base (A-). The weak acid readily donates a proton (H+), while the conjugate base accepts it, creating a buffer system that effectively neutralizes added acids.
-
Weak Base and Conjugate Acid: Conversely, these buffers comprise a weak base (B) and its conjugate acid (BH+). The weak base accepts protons, while the conjugate acid donates them, establishing a buffer system that efficiently neutralizes added bases.
The Significance of pKa
The pKa value plays a pivotal role in determining the effectiveness of a buffer. pKa represents the pH at which the concentration of the weak acid (HA) equals that of its conjugate base (A-). Buffers exhibit maximum effectiveness when the pH of the solution is within one pH unit of their pKa. This narrow pH range allows the buffer to neutralize both acids and bases without significant pH fluctuations.
pH Regulation by Biological Buffers
The Tale of pH Guardians
In the intricate symphony of life, pH plays a pivotal role, much like the conductor of an orchestra. Maintaining a stable internal pH is crucial for the harmonious functioning of biological processes. This delicate equilibrium is safeguarded by biological buffers, the unsung heroes of pH homeostasis.
Imagine a magical kingdom where acids and bases are constantly vying for dominance. Biological buffers step into this chaotic realm as valiant knights, protecting the pH balance with their unwavering resolve. Acids, the invading foes, are deflected by buffers, which convert them into less potent weak acids. This conversion effectively neutralizes the acid’s disruptive influence.
On the other side of the battlefield, bases, the insidious attackers, meet their match in buffers. Buffers stand their ground, transforming these bases into their weaker counterparts, conjugate acids. This clever strategy ensures that the pH remains within the optimal range.
A Deeper Dive into Neutralization
Let’s delve deeper into the chemical wizardry of buffer neutralization. When an acid infiltrates our biological kingdom, buffers swiftly engage in a chemical duel. The buffer’s conjugate base gallantly sacrifices itself to neutralize the invader, forming a weak acid. This sacrifice ensures that the pH remains relatively stable, shielding vital biological processes from disruption.
Conversely, when a base threatens the pH balance, buffers summon their weak acid allies. These weak acids neutralize the base, creating a conjugate acid. Once again, the buffer has thwarted the pH destabilization attempt, preserving the delicate equilibrium of life.
In this ongoing battle for pH stability, biological buffers tirelessly stand guard, ensuring that the symphony of life continues uninterrupted. Their unwavering dedication to pH homeostasis is the foundation upon which countless biological wonders can flourish.
Types of Biological Buffers
In the realm of living organisms, maintaining a stable pH balance is paramount for optimal functioning. Biological buffers, nature’s pH guardians, rise to this challenge by resisting changes in pH. Among the diverse array of buffers in the biological realm, three stand out as the primary protectors of pH homeostasis: carbonic acid/bicarbonate, phosphate, and protein buffers.
Carbonic Acid/Bicarbonate Buffer: The Guardian of Blood and Extracellular Fluids
The carbonic acid/bicarbonate buffer system reigns supreme in blood and extracellular fluids. At the heart of this defense mechanism lies carbonic acid (H2CO3), formed when carbon dioxide dissolves in water. In a delicate dance, carbonic acid readily dissociates into bicarbonate ions (HCO3-) and hydrogen ions (H+).
This buffer system shines when acids encroach. Hydrogen ions, the hallmark of acidity, are swiftly neutralized by bicarbonate ions, effectively curbing pH drops. Conversely, should bases threaten to raise pH levels, bicarbonate ions tag team with hydrogen ions to form carbonic acid, restoring the delicate pH balance.
Phosphate Buffer: The Cell’s Internal Guardian
Within the confines of cells, the phosphate buffer system holds sway. This buffer duo comprises dihydrogen phosphate (H2PO4-) and hydrogen phosphate (HPO42-). Their interplay mirrors that of the carbonic acid/bicarbonate buffer, with dihydrogen phosphate neutralizing excess hydrogen ions and hydrogen phosphate neutralizing hydroxide ions (bases).
The phosphate buffer system safeguards the intracellular environment, ensuring that the myriad of cellular processes can proceed smoothly within a stable pH range.
Protein Buffer: The Versatile Defender
Protein buffers, ubiquitous in both extracellular and intracellular environments, exhibit remarkable versatility in pH regulation. The secret lies in their amino acid side chains, which possess weak acidic or basic properties.
Under acidic conditions, protein buffers step up, offering their basic side chains to neutralize hydrogen ions. When bases rear their heads, acidic side chains come to the rescue, neutralizing hydroxide ions. This remarkable adaptability allows protein buffers to protect pH balance in a wide range of settings.
Buffer Capacity: The Resilience of Biological Buffers
Biological buffers are like stalwart guardians, vigilantly maintaining the delicate pH balance within our bodies. Amidst the constant ebb and flow of acids and bases, they stand firm, ensuring that the pH remains within a narrow, life-sustaining range.
Understanding Buffer Capacity
Buffer capacity measures the resilience of a buffer, its ability to resist pH changes. It’s analogous to a boxer’s ability to withstand punches without getting knocked down. The higher the buffer capacity, the more acids or bases it can neutralize without significantly altering the pH.
Factors Influencing Buffer Capacity
Two key factors influence buffer capacity: concentration and pKa.
Concentration: The higher the concentration of the buffer components (weak acid and conjugate base, or weak base and conjugate acid), the more acids or bases it can neutralize. Think of it like having more soldiers in your army; more soldiers can neutralize more enemy forces.
pKa: pKa is the pH at which the weak acid and its conjugate base are present in equal concentrations. The closer the pKa to the pH of the solution, the higher the buffer capacity. It’s like having a boxer who’s perfectly matched to his opponent; he can absorb more punches before being overwhelmed.
Buffer capacity is crucial for maintaining pH homeostasis. By understanding how concentration and pKa influence buffer capacity, we can appreciate the remarkable resilience of biological buffers in safeguarding the delicate pH balance essential for life.