Determining Concentration From Absorbance: A Comprehensive Guide Using The Beer-Lambert Law
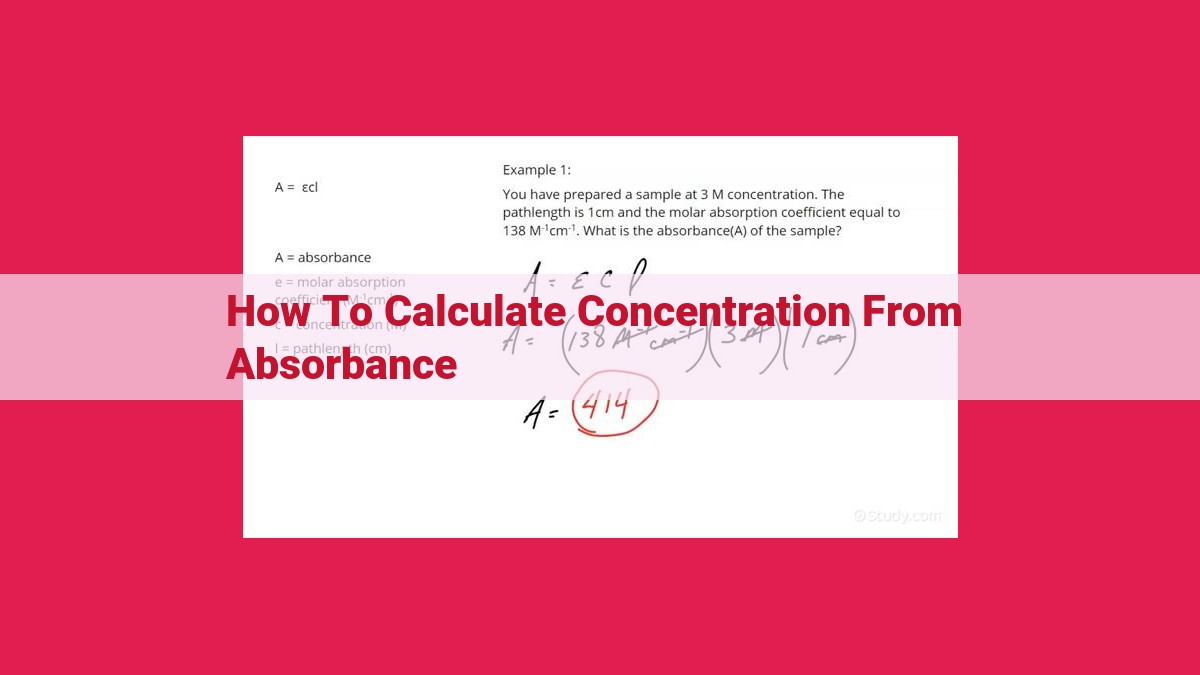
To determine concentration from absorbance, the Beer-Lambert Law (A = εbc) is used. Absorbance (A) measured at a specific wavelength is directly proportional to the concentration (c), path length (b), and molar absorptivity (ε). By obtaining the molar absorptivity through calibration using standard solutions, and measuring the absorbance of the sample, the concentration can be calculated. This method is widely used in spectrophotometry for quantifying the concentration of various substances, such as proteins, DNA, and ions, in biological, chemical, and industrial applications.
- Importance of concentration determination
- Spectrophotometry as a method for quantification
Understanding the Importance of Concentration Determination and Spectrophotometry
In various scientific and industrial fields, determining the concentration of substances is crucial for a range of applications. From evaluating the purity of products to monitoring environmental pollutants, precise concentration measurements are indispensable. Spectrophotometry, a widely used technique, plays a pivotal role in quantifying the concentration of solutions by analyzing the interaction between light and the sample.
Spectrophotometry utilizes the absorption or transmission of light by a sample to determine its concentration. When light passes through a substance, some of its energy is absorbed by the sample, leading to a decrease in the intensity of the transmitted light. The amount of absorption is directly proportional to the concentration of the substance in the sample. This fundamental principle forms the basis of spectrophotometry.
Key Concepts in Spectrophotometry for Concentration Determination
-
Absorbance: A measure of the decrease in light intensity due to absorption by the sample, providing a quantitative indication of the sample’s concentration.
-
Beer-Lambert Law: A fundamental relationship that establishes a linear connection between absorbance, concentration, path length (the distance light travels through the sample), and molar absorptivity (a constant for each substance at a specific wavelength).
-
Concentration: The amount of substance present per unit volume of a solution, commonly expressed in units such as molarity (moles per liter) or normality (equivalents per liter).
-
Path Length: The thickness of the sample through which light passes, affecting the amount of absorption.
-
Molar Absorptivity: A proportionality constant that represents the ability of a substance to absorb light at a particular wavelength.
-
Dilutions: The process of decreasing the concentration of a solution by adding more solvent, allowing for the accurate measurement of samples with high concentrations.
-
Standard Solutions: Solutions with precisely known concentrations, used as references for calibration and validation of spectrophotometric measurements.
Understanding the Concepts Behind Spectrophotometric Concentration Determination
Spectrophotometry: Unveiling the Secrets of Light Interaction
Spectrophotometry is a powerful analytical technique that harnesses the interaction of light with substances to determine their concentration. It’s a cornerstone of scientific research and industrial applications, enabling us to quantify the presence of specific molecules or ions.
Absorbance: Measuring Light Absorption
When light passes through a sample, some of it is absorbed. This absorbed light is quantified as absorbance, a logarithmic measure that ranges from zero to infinity. The higher the absorbance, the more light absorbed and the stronger the concentration of the target substance.
Beer-Lambert Law: Unraveling the Relationship between Light and Concentration
The Beer-Lambert Law establishes a fundamental relationship between absorbance, concentration, and path length. This formula tells us that absorbance is directly proportional to both concentration and path length.
Molar Absorptivity: The Substance’s Fingerprint
In this equation, molar absorptivity is a constant that quantifies the specific absorbance of a substance at a given wavelength. It’s like a unique fingerprint for each molecule, enabling us to identify and differentiate substances.
Concentration: The Key to Quantifying Substance Presence
Concentration, expressed in units such as molarity or normality, represents the amount of substance dissolved in a specific volume of solution. By measuring absorbance and utilizing the Beer-Lambert Law, we can determine the concentration of an unknown substance accurately.
Path Length: Measuring the Light’s Journey
Path length refers to the distance light travels through the sample. In spectrophotometry, this distance is typically measured through the use of cuvettes. Understanding path length is crucial for accurate concentration determination.
Dilutions: Adjusting Concentration for Enhanced Accuracy
To analyze substances with concentrations beyond the spectrophotometer’s detection range, dilutions are performed. Dilutions involve adding a known volume of solvent to the original sample, decreasing its concentration while maintaining a proportional relationship.
Standard Solutions: Ensuring Precision in Measurement
Standard solutions are solutions with precisely known concentrations. They serve as reference points for spectrophotometric measurements, enabling us to calibrate our equipment and ensure accurate concentration determination of unknown samples.
Procedure:
- Measuring absorbance at specified wavelength
- Obtaining molar absorptivity
- Determining path length
- Calculating concentration using Beer-Lambert Law formula
Calculate Concentration from Absorbance: Unraveling the Secrets of Spectrophotometry
In the realm of scientific inquiry and industrial applications, determining the concentration of substances is a crucial aspect. Spectrophotometry, a technique that employs the interaction of light with matter, offers a precise and versatile solution to this challenge. This blog post will delve into the concepts and procedure involved in calculating concentration from absorbance using spectrophotometry, making it accessible and relatable for all.
Concepts to Grasp:
- Absorbance: It measures the extent to which light is absorbed when passing through a sample, directly related to the concentration of the substance present.
- Beer-Lambert Law: This fundamental equation states the relationship between absorbance, concentration, path length, and molar absorptivity:
A = εbc. - Molar Absorptivity (ε): A compound-specific constant indicating the amount of light absorbed per unit concentration and path length.
- Path Length (b): The distance that light travels through the sample, often measured in centimeters.
Procedure: Deciphering the Steps
With these concepts in mind, let’s explore the step-by-step procedure for determining concentration:
-
Measuring Absorbance at a Specific Wavelength: Using a spectrophotometer, measure the absorbance of the sample at a wavelength that corresponds to the maximum absorption of the substance of interest. This value, denoted as A, is a measure of the intensity reduction of the transmitted light.
-
Obtaining Molar Absorptivity: If known, use the molar absorptivity value (ε) from reference sources or literature. Alternatively, it can be experimentally determined using a standard solution of known concentration.
-
Determining Path Length: The path length (b) is usually fixed and standardized in the spectrophotometer cell used for the measurement. Check the specifications of the cell for the exact path length value.
-
Calculating Concentration: Plug the values of A, ε, b into the Beer-Lambert Law equation:
Concentration (c) = A / (ε * b)to calculate the concentration of the substance in the sample.
The ability to calculate concentration from absorbance using spectrophotometry empowers researchers and professionals across various fields. From medical diagnostics to environmental monitoring and industrial chemistry, this technique plays a vital role in:
- Quantifying the concentration of known substances
- Identifying and characterizing unknown compounds
- Monitoring analyte levels in real-time processes
掌握 the concepts and procedure of spectrophotometric concentration determination opens doors to a world of scientific exploration and practical applications. Embrace the power of light to unravel the mysteries of matter and drive innovation in countless endeavors.