Battery Cells: The Basics Of Electricity Generation (Optimized For Seo)
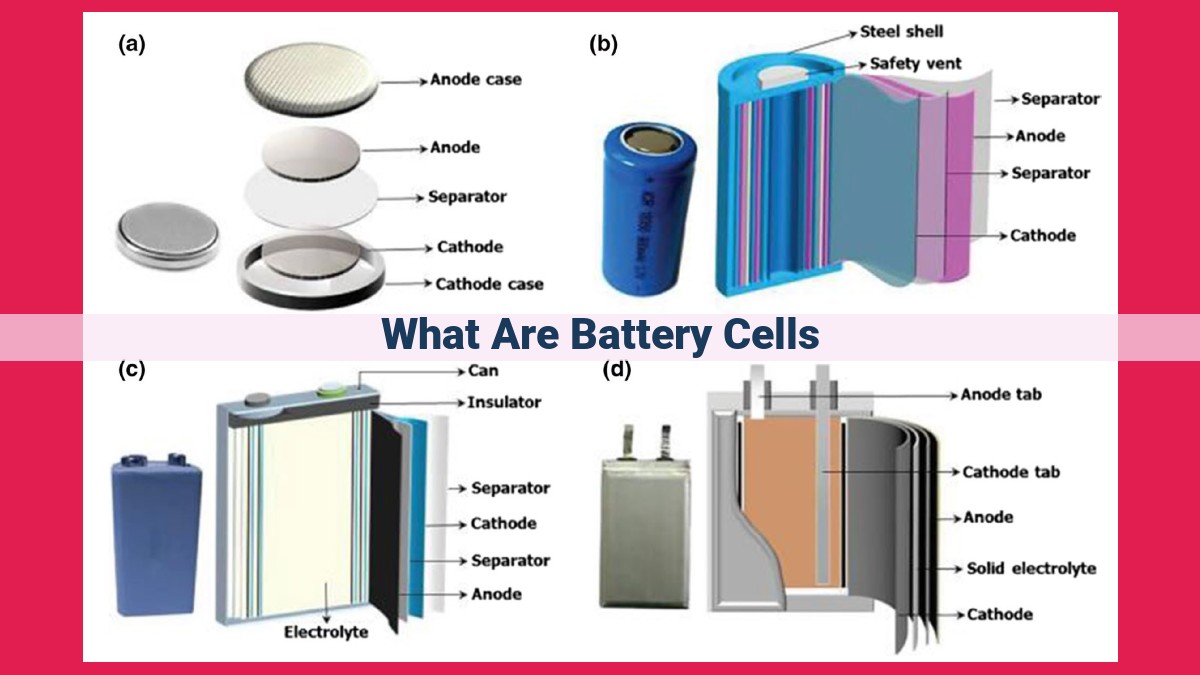
Battery cells, the fundamental units of electricity generation, consist of electrodes (anodes and cathodes), an electrolyte, and a separator. Anodes release ions and electrons, while cathodes receive them. The electrolyte enables ion movement, and the separator prevents short circuits. Key battery metrics include capacity, voltage, energy density, and power density. Multiple cells are interconnected to form a battery, and terminals facilitate connections. Understanding ions, oxidation, and reduction is essential for comprehending battery cell functioning.
Fundamentals of Battery Cells: The Powerhouses of Modern Life
In today’s fast-paced world, batteries have become essential to our daily lives, powering everything from smartphones to electric vehicles. But have you ever wondered about the inner workings of these portable powerhouses? Let’s dive into the fundamentals of battery cells to understand how they generate electricity and store energy.
The Basic Unit: The Battery Cell
A battery cell is the smallest unit of electricity generation. It consists of electrodes, electrolyte, and current collectors. The electrodes are two conducting materials, the anode (the negative electrode) and the cathode (the positive electrode). When immersed in the electrolyte, a liquid or paste that contains ions, a chemical reaction occurs between the two electrodes, releasing electrons and generating an electric current.
The Key Players: Electrodes and Current Collectors
The electrodes play a crucial role in the electrochemical process. The anode is typically made of a metal that can easily give up electrons (e.g., lithium), while the cathode is made of a material that can readily accept electrons (e.g., carbon, metal oxides). The movement of electrons from the anode to the cathode creates an electrical current.
Current collectors are metallic conductors that facilitate the flow of electrons between the electrodes and the external circuit. They ensure that the electrons released from the anode are efficiently transferred to the cathode to generate the electric current.
Types of Electrodes and Their Functions:
- Describe the distinction between an anode (negative electrode) and a cathode (positive electrode).
- Explain the electrochemical reactions that occur at each electrode.
Types of Electrodes and Their Functions
In the heart of every battery cell, electrodes dance the waltz of electrochemical reactions, orchestrating the flow of electrons and the generation of electricity. Two primary players grace this stage: the anode and the cathode.
The Anode: A Negative Player with a Positive Impact
The anode, the negative electrode, plays a crucial role in facilitating the chemical dance. Composed of a material that readily gives up electrons, such as carbon or lithium, the anode acts as the launching pad for electrons. During the discharge process, these electrons break free and embark on their journey through the external circuit, powering laptops, phones, and countless other devices.
The anode undergoes an electrochemical transformation known as oxidation, where it loses electrons and bonds with positively charged ions from the electrolyte. This reaction constantly replenishes the supply of electrons available for the external circuit, ensuring a steady flow of electricity.
The Cathode: A Positive Force in Energy Conversion
The cathode, the positive electrode, stands on the opposite end of the electrochemical spectrum. Crafted from a material that has an affinity for electrons, such as metal oxides or oxygen, the cathode eagerly accepts the electrons that come its way.
During discharge, the cathode undergoes a process called reduction, where it gains electrons and reacts with negative ions in the electrolyte. This reaction consumes the electrons flowing from the anode, thereby completing the circuit and generating electrical energy.
A Delicate Balance of Electrochemical Reactions
The anode and cathode, working in tandem, create an electrochemical waltz where electrons flow with finesse and chemical reactions unfold with precision. The interplay of oxidation and reduction fuels the battery cell’s ability to generate electricity on demand.
Their distinct roles and opposing reactions are essential for the continuous flow of electrons that powers our modern world, from the smartphones in our hands to the electric vehicles gliding on our roads.
Electrolyte and Separator: Ionic Conductors and Circuit Protection
In the heart of every battery cell lies a crucial duo: the electrolyte and the separator. Together, these components play a vital role in regulating the flow of electricity and ensuring the safety and longevity of your battery.
The Electrolyte: Conduit for Ionic Movement
Imagine the electrolyte as a busy highway for charged particles, known as ions. These ions are like tiny commuters that carry electrical charge. The electrolyte is a substance that allows these ions to move freely within the battery cell. By providing a pathway for ion movement, the electrolyte enables the flow of electricity between the electrodes.
The Separator: Guardian of Circuit Integrity
Just as traffic signals prevent collisions on a busy street, the separator in a battery cell serves as a safety barrier. It prevents direct contact between the positive and negative electrodes, known as a short circuit. This is crucial because a short circuit would allow uncontrolled current flow, leading to rapid battery discharge and potential damage.
Key Takeaways:
- The electrolyte is the medium that allows ions to move within the battery cell, facilitating the flow of electricity.
- The separator acts as a barrier, preventing short circuits and ensuring the safe and stable operation of the battery.
Key Battery Performance Metrics: Unlocking Battery Efficiency
Understanding battery performance is crucial in our increasingly reliant world on devices and vehicles powered by these electrochemical marvels. Among the myriad metrics used to evaluate battery capabilities, capacity, voltage, energy density, and power density stand out as essential indicators of a battery’s abilities.
Capacity: The Battery’s Storage Prowess
Capacity measures the total amount of electrical charge a battery can store. It’s analogous to the size of a fuel tank in a car, determining how long the battery can power devices before needing a recharge. Capacity is expressed in ampere-hours (Ah), representing the number of hours a battery can deliver one ampere of current.
Voltage: The Battery’s Electrical Potential
Voltage is the electrical potential difference between the battery’s terminals. It represents the force that drives electrons through the circuit, akin to the pressure in a water pump. Voltage is measured in volts (V) and indicates the battery’s ability to push current through a load.
Energy Density: Packing Power into a Compact Space
Energy density quantifies how much energy a battery can store relative to its weight or volume. A battery with high energy density can deliver more energy without being bulky or heavy. This metric is crucial for portable devices and electric vehicles, where space and weight are at a premium. Energy density is expressed in watt-hours per kilogram (Wh/kg) or watt-hours per liter (Wh/L).
Power Density: Unleashing Power When You Need It
Power density measures the amount of power a battery can deliver relative to its weight or volume. It indicates the battery’s ability to output current quickly. A battery with high power density can power high-power devices and support fast charging. Power density is expressed in watts per kilogram (W/kg) or watts per liter (W/L).
Electrical Connections and Battery Assembly: Making the Power Flow
When you think of batteries, imagine tiny powerhouses filled with electrochemical energy, ready to light up your devices or keep your car running. But before that energy can be harnessed, it must be properly connected and assembled.
The Role of Terminals: Gateway to Energy Flow
Every battery cell has two terminals: the positive terminal and the negative terminal. These terminals act as the gateways for electricity to flow in and out of the cell. The positive terminal is typically marked with a plus sign (+) while the negative terminal is marked with a minus sign (-).
Interconnecting Cells: Building a Collective Power Source
To create a battery with more capacity or voltage, multiple cells are interconnected. This is where terminals play a crucial role. The positive terminal of one cell is connected to the negative terminal of the next cell, and so on. This daisy-chain arrangement allows the cells to work together as a cohesive power source.
Battery Assembly: Putting It All Together
Once the cells are interconnected, they are assembled into a battery pack or module. This involves securing the cells in a specific configuration and connecting them with internal wiring. The terminals of the battery pack are then connected to the device that needs power.
Electrical connections and battery assembly are essential steps in harnessing the power of electrochemical cells. By connecting cells with terminals and assembling them properly, we create a reliable and efficient source of energy that powers our everyday lives.
Related Electrochemical Concepts:
- Introduce ions as charged particles involved in electrochemical reactions.
- Define oxidation and reduction as fundamental electrochemical processes.
Fundamentals of Battery Cells: The Building Blocks of Electrical Energy
At the heart of all battery technologies lies the battery cell, the fundamental unit of electricity generation. Each cell comprises electrodes, electrolytes, and current collectors, working harmoniously to facilitate the flow of electrons. The anode (negative electrode) and cathode (positive electrode) undergo electrochemical reactions, releasing electrons that travel through an external circuit, creating an electrical current. The electrolyte provides a medium for ions to move between the electrodes, while the current collectors gather and distribute these electrons.
Types of Electrodes and Their Electrochemical Contributions
The anode and cathode play distinct roles in the battery’s electrochemical reactions. The anode acts as the oxidation site, where ions lose electrons and become oxidized. Conversely, the cathode serves as the reduction site, where ions gain electrons and undergo reduction. These reactions generate an electrical potential difference, the driving force behind the battery’s ability to power devices.
Electrolyte and Separator: Ion Highway and Circuit Guardians
The electrolyte, typically a liquid or gel, enables the movement of ions between the electrodes. It facilitates the transfer of positive ions (cations) towards the cathode and negative ions (anions) towards the anode, completing the circuit. The separator, a porous material placed between the electrodes, plays a crucial role in preventing short circuits while allowing ion flow, ensuring the battery’s safe and efficient operation.
Key Battery Performance Metrics: Quantifying Battery Capacity and Efficiency
Several metrics quantify a battery’s performance: capacity, voltage, energy density, and power density. Capacity measures the amount of charge a battery can store, expressed in Ampere-hours (Ah). Voltage represents the electrical potential difference between the electrodes, determining the battery’s operating voltage. Energy density indicates the amount of energy stored per unit mass or volume, a key factor for applications such as electric vehicles and portable electronics. Power density measures the battery’s ability to deliver power quickly, crucial for high-power applications.
Electrical Connections and Battery Assembly: Creating a Power Source
Terminals provide the electrical connections to the battery, allowing the flow of current into and out of the cell. Multiple cells can be interconnected in series (positive to negative) or parallel (positive to positive, negative to negative) to achieve the desired voltage and capacity. This flexibility allows batteries to be tailored to specific applications, from small electronic devices to large-scale energy storage systems.
Related Electrochemical Concepts: The Underlying Processes
The ions involved in battery reactions are electrically charged particles. Electrochemical reactions involve oxidation, the loss of electrons from ions at the anode, and reduction, the gain of electrons by ions at the cathode. Understanding these fundamental processes is essential for comprehending the workings of battery cells and their applications in the modern world.