Basic Solutions: Definition, Properties, Examples, Applications, And Safety
- Definition of Basic Solutions
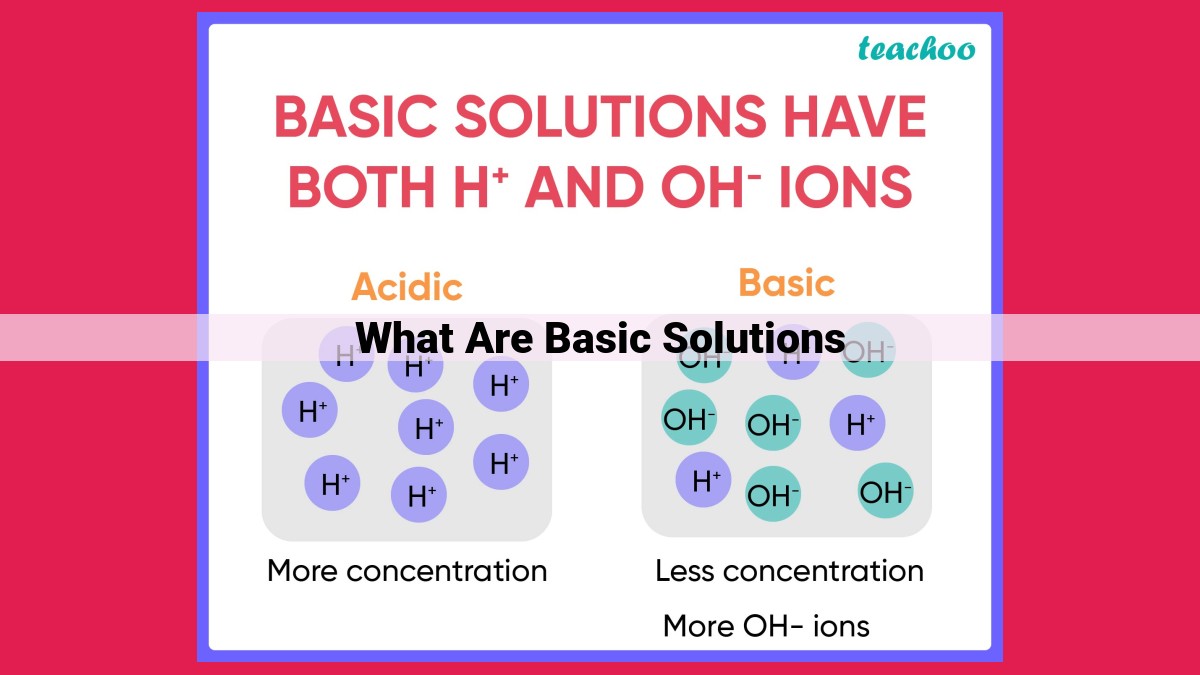
- Basic solutions are solutions with a pH greater than 7 and contain a higher concentration of hydroxide ions (OH-) than hydrogen ions (H+). They are considered alkaline and have the opposite properties of acidic solutions.
- Properties of Basic Solutions
- Basic solutions typically have a pH range of 7 to 14, are slippery to the touch, have a bitter taste, and turn litmus paper blue. They can also be corrosive and react with acids to form salts.
- Common Examples of Basic Solutions
- Common basic solutions include household ammonia, sodium hydroxide, and potassium hydroxide. These solutions are used in various applications, such as cleaning, degreasing, and manufacturing.
- Applications of Basic Solutions
- Basic solutions are used in a wide range of industries, including cleaning products, fertilizers, paper manufacturing, and pharmaceuticals. They can also be used as antacids and in water treatment.
- Safety Precautions
- Basic solutions can be corrosive and harmful to the skin and eyes. Proper protective equipment, such as gloves and goggles, should be worn when handling them.
Understanding the Essence of Basic Solutions: A Journey into the Realm of Chemistry
In the realm of chemistry, the concept of basic solutions holds a profound significance. Basic solutions, also known as alkaline solutions, possess a unique set of properties that distinguish them from their acidic counterparts. They are characterized by their ability to neutralize acids, forming salts and water. Their pH value, a measure of their acidity or alkalinity, typically ranges from 7 to 14, with 7 being neutral.
The connection between pH, acidity, and alkalinity forms an intricate web. Acidity is characterized by a pH below 7, indicating a higher concentration of hydrogen ions (H+). Alkalinity, on the other hand, is associated with a pH above 7, denoting a lower concentration of hydrogen ions. Neutrality, with a pH of 7, represents a balance between hydrogen ions and hydroxide ions (OH-).
**Unveiling the Intriguing Properties of Basic Solutions**
In the realm of chemistry, basic solutions occupy a captivating space, embodying unique properties that set them apart. These solutions possess a pH range that typically exceeds 7, indicating their alkaline nature. This characteristic has far-reaching implications, influencing their behavior and applications.
Immerse yourself in their slippery texture, a sensation that arises from the presence of hydroxide ions. These ions interact with your skin, forming a thin layer that reduces friction. Basic solutions also exhibit a bitter taste, a testament to their high pH levels.
The impact of basic solutions on litmus paper is unmistakable. This indicator, renowned for its ability to distinguish between acids and bases, turns blue in the presence of basic solutions. This color change serves as a telltale sign, revealing the alkaline nature of the solution.
Common Examples of Basic Solutions
In the realm of chemistry, basic solutions play a crucial role due to their unique properties and wide-ranging applications. These solutions, characterized by a pH value greater than 7, exhibit a remarkable array of characteristics that distinguish them from acidic and neutral solutions.
One prime example of a basic solution is household ammonia (NH3). This colorless gas, commonly encountered as an aqueous solution, possesses a distinctive pungent odor and a slippery feel. Its alkalinity makes it a versatile cleaning agent, effective in removing grease and grime.
Another prominent basic solution is sodium hydroxide (NaOH), also known as lye. This white, crystalline solid is highly corrosive and soluble in water, releasing heat upon dissolution. Sodium hydroxide finds extensive use in industries, particularly in the production of soaps, detergents, and textiles.
Potassium hydroxide (KOH), akin to sodium hydroxide, is a strong base with a wide range of applications. This white, deliquescent solid is employed in the production of fertilizers, batteries, and pharmaceuticals.
These basic solutions, with their distinct properties and uses, exemplify the versatility and significance of this category in various scientific and industrial domains.
Applications of Basic Solutions: A Versatile Tool in Everyday Life and Industry
Basic solutions, with their unique properties, find widespread applications in various aspects of our daily lives and industrial processes.
Cleaning and Degreasing
The effectiveness of basic solutions in cleaning and degreasing is widely recognized. Their ability to neutralize acids and dissolve grease makes them ideal for removing dirt, oil, and grime from surfaces. Household ammonia, with its characteristic pungent odor, is a common example used in cleaning floors, windows, and bathrooms.
Industrial Applications
Beyond household cleaning, basic solutions play a crucial role in industrial settings. In the manufacturing of glass, ceramics, and textiles, basic solutions are used as fluxing agents, helping to melt and fuse materials together. They also find application in the production of paper, soaps, and detergents.
Sodium hydroxide, a strong base, is particularly versatile in industrial processes. It is used in the production of rayon, soap, and paper. Potassium hydroxide, another strong base, is commonly employed in the manufacture of potash fertilizers and electrolytic cells.
Safety Precautions for Handling Basic Solutions
While basic solutions are valuable tools, they can also pose safety hazards. It is important to handle them with caution. Strong bases, in particular, are corrosive and can cause severe burns on contact with skin.
When working with basic solutions, it is essential to wear appropriate personal protective equipment, such as gloves, goggles, and a lab coat. Avoid direct contact with skin and eyes, and handle spills immediately. Proper ventilation is also crucial to prevent inhalation of harmful fumes.
Basic solutions are versatile and indispensable in a wide range of applications, from household cleaning to industrial processes. Their ability to neutralize acids, dissolve grease, and alter the pH of solutions makes them essential tools in our daily lives and various industries. However, it is important to exercise caution when handling basic solutions and to always prioritize safety.
Safety Precautions for Handling Basic Solutions
The Corrosive Nature of Basic Solutions
Basic solutions possess a corrosive nature, meaning they can cause damage to various surfaces and even living tissues. This is due to their high pH levels, which promote the breakdown of organic materials. It is crucial to handle basic solutions with utmost care to minimize potential hazards.
Guidelines for Safe Handling
To ensure the safety of individuals handling basic solutions, it is imperative to adhere to the following guidelines:
- Wear appropriate personal protective equipment (PPE): This includes gloves, eye protection (goggles or face shield), and a lab coat to prevent contact with the solution.
- Handle solutions in a well-ventilated area: Fumes emitted from basic solutions can irritate the respiratory system. Ensuring proper ventilation helps mitigate this risk.
- Avoid direct contact with skin and eyes: Basic solutions can cause severe burns upon contact. If exposed, flush the affected area thoroughly with water and seek medical attention immediately.
- Store solutions properly: Keep basic solutions in secure and well-labeled containers to prevent spills and accidents. Dispose of solutions responsibly in accordance with local regulations.
By diligently following these safety precautions, you can minimize the risks associated with handling basic solutions and maintain a safe working environment.