Unraveling Base Strength: A Comprehensive Guide For Chemistry, Titrations, And Catalysis
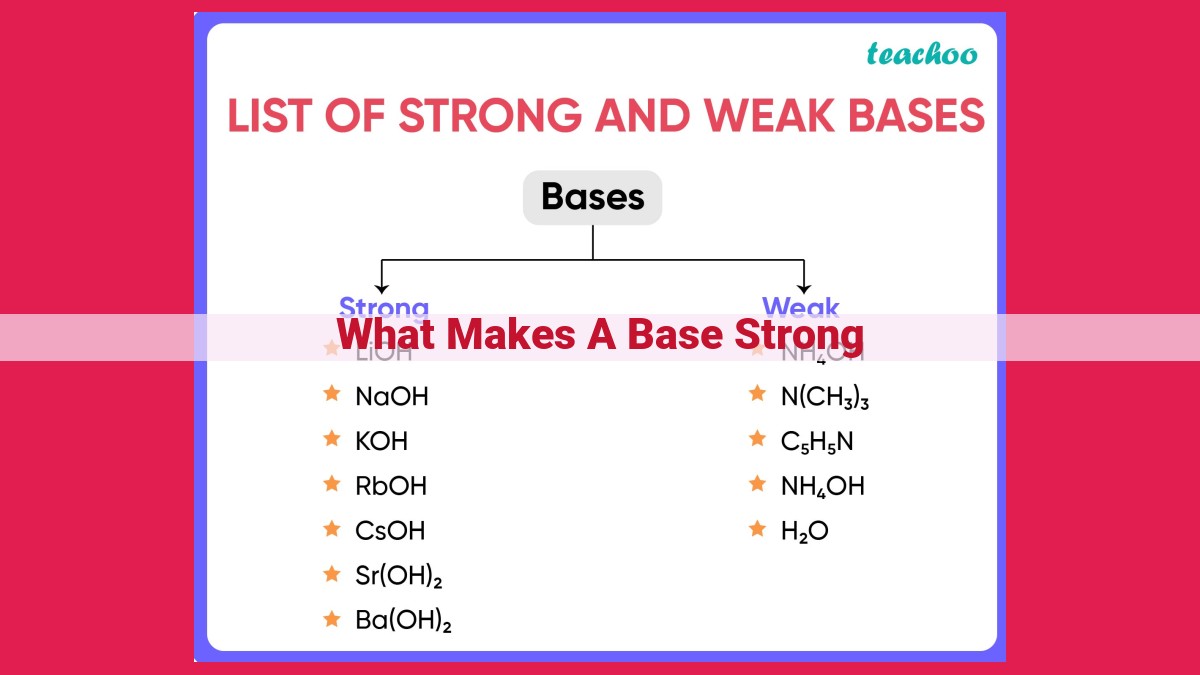
A strong base is characterized by its ability to readily lose hydroxide ions (OH-) in water. Its strength is determined by several factors: pKa of the conjugate acid (lower pKa indicates stronger base), solubility in water (weak bases dissolve more easily), dissociation constant (lower pKb indicates stronger base), strength of the conjugate acid (strong base has a weak conjugate acid), hydration energy of the conjugate base (larger and more charged conjugate base has higher hydration energy), resonance stabilization of the conjugate base (more resonance structures enhance base stability), and inductive effect of substituents (electronegative substituents weaken bases). Understanding base strength is crucial in chemistry, acid-base titrations, and catalysis.
Understanding the Power of Strong Bases
In the vast realm of chemistry, understanding the strength of bases is crucial. Strong bases possess a remarkable ability to completely dissociate in water, releasing hydroxide ions (OH-) and playing a vital role in numerous fields.
From industrial processes to biological systems, strong bases contribute significantly. They are employed in the production of soaps and detergents, neutralizing acids, and regulating pH levels in biological fluids. Their versatility extends to electrochemical cells, where they serve as electrolytes.
Recognizing the significance of strong bases, chemists have developed quantitative measures, such as the dissociation constant (Kb), to determine their strength. A lower pKb value indicates a stronger base, as it corresponds to a higher concentration of hydroxide ions in solution.
pKa of Conjugate Acid: Unraveling the Interplay of Acid-Base Strength
In the realm of chemistry, the strength of a base is not an isolated concept. It is intricately linked to the pKa value of its conjugate acid. Allow us to unravel this captivating relationship, revealing the profound impact it holds on base strength and the formation of conjugate bases.
The Inverse Correlation: A Dance of Opposites
The pKa of a conjugate acid exhibits an inverse relationship with the strength of its corresponding base. Simply put, a stronger base possesses a weaker conjugate acid, and vice versa. This intimate connection stems from the fundamental nature of acid-base chemistry.
Stronger bases readily accept protons, effectively weakening the ability of their conjugate acids to donate protons. On the other hand, weaker bases release protons more easily, rendering their conjugate acids stronger proton donors.
Solution pH: The Orchestrator of Conjugate Base Formation
The pH of a solution plays a pivotal role in the formation of conjugate bases. In acidic solutions (low pH), the proton concentration is high, favoring the protonation of bases and suppressing the formation of conjugate bases. Conversely, in basic solutions (high pH), the proton concentration is low, allowing bases to accept protons and promoting the formation of conjugate bases.
Consider the equilibrium reaction:
Base + H+ <=> Conjugate Acid
In acidic solutions, the equilibrium shifts towards the left, favoring the formation of the conjugate acid. In basic solutions, the equilibrium shifts towards the right, leading to the formation of the conjugate base.
The pKa value of a conjugate acid and the strength of its corresponding base are intertwined, like two sides of the same coin. A strong base arises from a weak conjugate acid, and vice versa. The interplay between solution pH and acid-base strength governs the formation of conjugate bases, opening up avenues for intricate chemical reactions.
Solubility of Bases in Water
Understanding the solubility of bases in water is crucial for various chemical and everyday applications. Several factors, including polarity and molecular size, influence how readily a base dissolves in water.
Polarity and Base Solubility
Bases, especially weak bases, tend to be more polar in nature. This means that they have regions of partial positive and negative charges within their molecules. When a polar base comes into contact with water, the partial negative charge on the base molecule is attracted to the partial positive charge on the water molecules, leading to the formation of hydrogen bonds. These hydrogen bonds create a stable interaction between the base and water molecules, enhancing the solubility of the base.
Molecular Size and Solubility
Another factor that affects base solubility is molecular size. Smaller base molecules tend to be more soluble in water than larger ones. This is because smaller molecules can more easily penetrate the water molecules and form hydrogen bonds. As the molecular size increases, the number of hydrogen bonds that can form decreases, making the base less soluble.
In summary, polarity and molecular size play crucial roles in determining the solubility of bases in water. The presence of polar groups within a base molecule enhances hydrogen bonding with water, increasing its solubility. Smaller base molecules with greater surface area for hydrogen bonding exhibit higher solubility compared to larger ones.
The Dissociation Constant (Kb): A Measure of Base Strength
Imagine yourself at a party with a group of friends. Among them is a particularly outgoing and talkative individual who easily mingles with everyone. This person represents a strong base, a chemical species that readily accepts protons (H+ ions).
Just as the talkative friend effortlessly interacts with others, strong bases eagerly bind protons. This ability is quantified by a parameter known as the dissociation constant (Kb). The Kb value is a measure of the base strength, indicating how effectively a base can capture protons.
Strong bases possess a low Kb value, meaning they dissociate extensively in solution, releasing a large number of hydroxide ions (OH-). Think of it as the talkative friend effortlessly engaging with multiple people simultaneously.
Conversely, weak bases have a high Kb value, indicating limited proton acceptance and low dissociation in solution. They are like the shy individuals at the party, hesitantly interacting with others.
The Kb value not only measures base strength but also provides valuable insights into conjugate acid strength. A strong base will have a weak conjugate acid, characterized by a high pKa value, the measure of acid strength.
So, in the context of our party analogy, a strong base is akin to a popular friend who makes everyone around them feel at ease, while its conjugate acid is like the awkward guest who struggles to engage.
Understanding the dissociation constant (Kb) is crucial in many areas of chemistry. It helps us predict the pH of solutions, perform acid-base titrations, and design catalysts for various chemical reactions. By comprehending the interplay between Kb and base strength, we gain a deeper understanding of the complex world of chemistry.
The Intriguing Relationship Between Strong Bases and Their Conjugate Acids
In the realm of chemistry, we encounter substances known as bases, which play a pivotal role in countless reactions and applications. Among these bases, strong bases possess a distinct characteristic: they readily donate protons, making them essential in various fields.
To grasp the concept of a strong base, we must delve into the notion of conjugate acids. Every acid has a corresponding conjugate base, and vice versa. The strength of an acid and its conjugate base are inversely proportional. This means that strong bases have weak conjugate acids.
A strong base will readily accept a proton, thus shifting the equilibrium towards the formation of a weak conjugate acid. This is reflected in the high pKa value of the conjugate acid, indicating that it readily donates protons. Conversely, a weak base results in a strong conjugate acid with a low pKa value.
It’s crucial to understand the implications of this relationship. A strong base, with its ability to readily donate protons, can neutralize even strong acids, leading to the formation of a weak conjugate acid. This phenomenon is vital in maintaining pH balance in various systems, including our own bodies.
Hydration Energy of Conjugate Bases: The Stabilizing Force
In the realm of chemistry, bases play a pivotal role in numerous reactions. When a base dissolves in water, it undergoes a dissociation process, releasing hydroxide ions (OH-) and forming a conjugate acid. The strength of a base, measured by its dissociation constant (Kb), determines the extent to which it dissociates in solution. One key factor influencing base strength is the hydration energy of its conjugate base.
Hydration, the Energy-Saving Bond
Hydration refers to the process of surrounding an ion or molecule with water molecules. This interaction is driven by electrostatic forces between the ions and the polar water molecules. When a conjugate base forms, it carries a net negative charge and becomes surrounded by a shell of water molecules. These water molecules form hydrogen bonds with the conjugate base, stabilizing it and reducing its overall energy.
Size and Charge Matter
The hydration energy of a conjugate base is directly influenced by its size and charge. Larger conjugate bases tend to have higher hydration energies because they can accommodate more water molecules in their hydration shells. Highly charged conjugate bases also exhibit stronger hydration energies due to the increased electrostatic interactions with water molecules.
Implications of Hydration Energy
The hydration energy of a conjugate base has significant implications for base strength. Stronger hydration energies stabilize the conjugate base, making the base less likely to dissociate and release hydroxide ions. This results in a lower dissociation constant (Kb) and a weaker base. Conversely, weaker hydration energies destabilize the conjugate base, promoting dissociation and increasing base strength.
In summary, the hydration energy of a conjugate base is a crucial factor in determining base strength. Larger, highly charged conjugate bases possess stronger hydration energies, which stabilizes them and reduces their dissociation tendency. This results in weaker bases. Conversely, smaller, less charged conjugate bases have weaker hydration energies, which destabilizes them and enhances dissociation, leading to stronger bases. Understanding the role of hydration energy provides insights into the behavior of bases and their applications in various chemical processes.
Understanding the Essence of Resonance Stabilization in Base Strength
In the realm of chemistry, the strength of a base is a key concept that governs its reactivity and behavior. Resonance stabilization, a crucial factor in determining base strength, deserves special attention. Let’s dive into this fascinating phenomenon to unravel its impact on base stability.
When a conjugate acid donates a proton (H+), it forms a conjugate base. This conjugate base might possess multiple resonance structures, where the negative charge is delocalized over several atoms within the molecule. This delocalization of negative charge plays a significant role in enhancing base stability.
Resonance stabilization occurs when multiple resonance structures of a molecule have similar energies. Each resonance structure contributes to the overall stability of the molecule, and the more resonance structures there are, the more stable the molecule becomes.
In the case of a conjugate base, multiple resonance structures allow for the negative charge to be dispersed over a wider area, reducing the electron density at any one atom. This decrease in electron density makes the conjugate base less reactive and, therefore, a stronger base.
To illustrate this concept, let’s consider the example of acetate ion (CH3COO-), the conjugate base of acetic acid. Acetate ion can adopt two resonance structures, where the negative charge is located on either of the two oxygen atoms. This resonance stabilization significantly enhances the stability of acetate ion, making it a stronger base than it would be if it had only one resonance structure.
In summary, resonance stabilization of the conjugate base is a crucial factor in determining the overall strength of a base. By understanding this phenomenon, we can gain deeper insights into the behavior and reactivity of bases in various chemical systems.
The Power of Substituents: How They Control Base Strength
In the world of chemistry, nothing exists in isolation. Every molecule is influenced by its surroundings, including substituents – groups of atoms attached to the main structure. When it comes to bases, substituents play a crucial role in determining their strength.
Electronegativity Matters
Electronegativity is the ability of an atom to attract electrons. When an electronegative substituent is attached to a base, it pulls electrons away from the base’s conjugate acid. This makes the acid weaker and the base stronger.
Distance Matters Too
The distance between the substituent and the reaction site also affects its inductive effect. The closer the substituent is to the acid, the greater its influence. This is because the electrons have a shorter path to travel when pulled away.
Applications in Everyday Life
Understanding the inductive effect of substituents has practical implications in many areas, including:
- pH Control: Adjusting the pH of a solution requires an understanding of how substituents affect base strength.
- Acid-Base Titrations: Determining the equivalence point in an acid-base titration relies on the predictable behavior of bases with different substituents.
- Catalysis: Enzymes, biological catalysts, use the inductive effect of substituents to enhance their efficiency.
By mastering the concept of the inductive effect of substituents, you unlock a deeper understanding of base strength and its impact on chemical reactions and real-world applications.
Understanding Base Strength: Its Significance and Applications
Grasping the concept of base strength is crucial in various scientific and everyday scenarios. By understanding the properties that govern base strength, we gain insights into their practical applications and their impact on our world.
One practical application of understanding base strength is in pH control. The strength of a base determines its ability to neutralize acids and regulate the pH of solutions. This knowledge is essential in industries that rely on precise pH control, such as wastewater treatment, pharmaceuticals, and food processing.
Another important application lies in acid-base titrations, a technique used to determine the concentration of an unknown acid or base. The strength of the base used in the titration influences its ability to react completely with the acid, providing accurate and reliable concentration measurements.
Moreover, base strength plays a significant role in catalysis, a process that speeds up chemical reactions. Strong bases can act as catalysts in a variety of reactions, including hydrolysis and esterification. Understanding the strength of a base is therefore essential for optimizing reaction rates and yields in chemical processes.
In conclusion, understanding base strength is not only a fundamental concept in chemistry but also a practical tool with applications in diverse fields. From pH control to catalysis, base strength has a profound impact on our everyday lives and scientific endeavors. By unraveling its intricacies, we empower ourselves to harness the power of bases for a wide range of beneficial purposes.