Back Titration: A Comprehensive Guide To Enhanced Precision And Accuracy
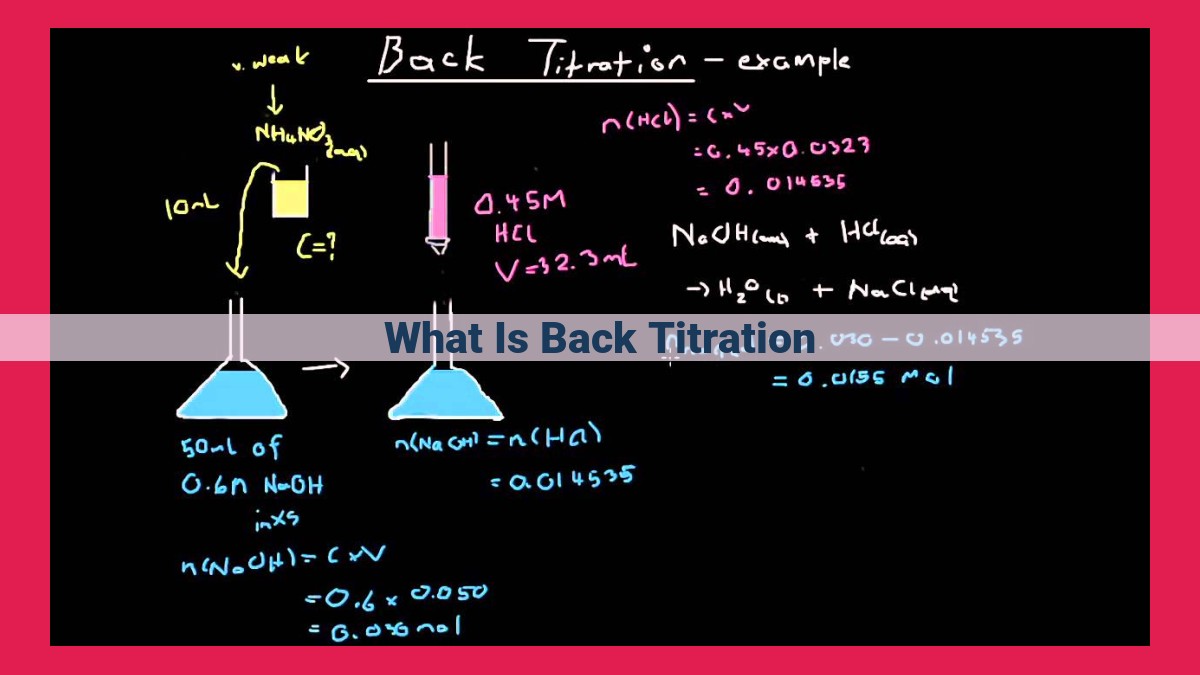
Back titration is a technique where an excess of reagent is added to a sample, followed by titration of the remaining excess reagent. It differs from traditional methods where the analyte is directly titrated. Back titration is beneficial when the analyte undergoes slow or incomplete reactions. The excess reagent ensures complete reaction and improves accuracy and precision. Applications include acid-base titrations, where it allows for the determination of weak acids or bases. Back titration offers advantages such as high accuracy and precision but can be complex and time-consuming compared to traditional titrations.
Back Titration: Delving into the Art of Indirect Titrations
In the realm of chemistry, titrations reign supreme as a cornerstone technique for analyzing substances. But what if the target substance is a bit shy and refuses to react directly? Enter the enigmatic world of back titration, a clever workaround that unveils hidden truths.
What is Back Titration?
Imagine a mischievous chemist adding an excess of reagent to a sample, creating a surplus of known concentration. Back titration comes to the rescue by introducing another reagent that reacts with this excess, consuming it until the reaction reaches a halt. By observing the amount of the second reagent used, we can deduce the original amount of analyte hiding within the sample. It’s like a chemical detective story, where the trail leads us to the target’s identity.
Why Back Titration?
Traditional titrations have their limits. Some substances are slowpokes, taking their sweet time to react. Others may have a mind of their own, reacting only partially. But back titration doesn’t care about their stubbornness. It bypasses these obstacles by targeting the excess reagent, making it an ideal choice for these reluctant reactants.
Accuracy and Precision: The Holy Grail of Titrations
Back titration shines when it comes to accuracy and precision. By neutralizing the excess reagent, we eliminate potential errors caused by incomplete reactions. This meticulous approach ensures that every drop of titrant counts, leading to reliable and reproducible results.
Real-World Applications: Where Back Titration Flexes Its Muscles
Back titration isn’t just a lab curiosity. It plays a vital role in various industries, including:
- Medicine: Analyzing drug concentrations in blood and other fluids
- Manufacturing: Monitoring chemical reactions and ensuring product quality
- Environmental Science: Measuring acidity or alkalinity in water and soil
Advantages and Disadvantages: Weighing the Pros and Cons
- Advantages:
- Accurate and precise results
- Overcomes slow or incomplete reactions
- Disadvantages:
- Can be more complex than traditional titration
- Requires careful preparation and execution
Back titration is a valuable tool in the chemistry toolbox, offering a clever way to determine the concentration of substances that prefer to play hide-and-seek. By embracing the power of indirect titration, we can uncover the secrets of these elusive compounds and gain a deeper understanding of the chemical world around us.
Difference from Traditional Titration
- Explain how back titration differs from the traditional titration method, where analyte is directly titrated.
Back Titration: A Different Approach to Titration
Unveiling the Difference: Back Titration vs. Traditional Titration
In the realm of chemistry, titration plays a crucial role in determining the concentration of an unknown substance. While traditional titration remains a fundamental technique, back titration offers a distinct approach that enhances accuracy and precision, particularly when dealing with slow or incomplete reactions.
Unlike traditional titration, where the analyte is directly titrated with a reagent, back titration involves adding an excess of known reagent to the sample. This excess reagent reacts with the analyte to form a new product. Subsequently, the remaining excess reagent is titrated with a different reagent of known concentration to determine the exact amount of excess reagent present.
This indirect measurement of the analyte’s concentration provides several advantages over traditional titration:
-
Enhanced Sensitivity: By using an excess of known reagent, back titration amplifies the sensitivity of the analysis. This becomes especially valuable when the analyte has a low concentration or the reaction between the analyte and the reagent is slow.
-
Improved Accuracy: The titration of the excess reagent avoids errors associated with the determination of the equivalence point in traditional titration, leading to more accurate results.
-
Increased Precision: Back titration allows for multiple titrations to be performed on a single sample, providing more data points and reducing random errors.
By combining these advantages, back titration establishes itself as a powerful tool for precise and accurate determination of analyte concentrations, especially when confronted with challenging reactions.
Back Titration: Unveiling Its Purpose in Analytical Chemistry
Back Titration – A Reverse Approach to Chemical Analysis
Back titration is a technique that turns the traditional titration method on its head. Instead of directly titrating the analyte, back titration involves adding an excess of a known reagent to the sample. This excess reagent then reacts with the analyte, and the remaining unreacted reagent is determined by a titration with a second reagent.
Unlocking the Secrets of Slow and Incomplete Reactions
Back titration comes to the rescue when dealing with analytes that reluctantly undergo chemical reactions or take their sweet time to reach completion. By adding an excess of reagent, back titration ensures that all of the analyte is converted into the product. The unreacted excess reagent is then titrated, allowing for an accurate determination of the analyte’s concentration.
Precision and Accuracy – The Holy Grail of Analytical Chemistry
Back titration shines when it comes to precision and accuracy. By titrating the excess reagent rather than the analyte, it minimizes errors from incomplete reactions. This approach ensures that even the most stubborn analytes are accurately quantified, making back titration a reliable technique for complex chemical analyses.
Back Titration: Unlocking Accuracy and Precision in Chemical Analysis
In the world of chemistry, precision and accuracy are essential for reliable results. Traditional titration methods often face challenges when dealing with reactions that are slow or incomplete. Enter back titration, a technique that has revolutionized the realm of chemical analysis.
Back titration stands apart from its traditional counterpart by its unique approach. Instead of directly titrating the analyte, it involves adding an excess of reagent to the sample and then titrating the unreacted excess.** This ingenious strategy overcomes the limitations of traditional methods, delivering unparalleled accuracy and precision.**
The accuracy of back titration stems from its ability to account for all the reactants involved in the reaction. By titrating the excess reagent, it ensures complete consumption of the analyte, eliminating any errors due to incomplete reactions or slow kinetics.** Additionally, the use of a known excess of reagent allows for precise control over the reaction stoichiometry, further enhancing the accuracy of the results.**
Precision, the reproducibility of measurements, is another hallmark of back titration. The excess of reagent ensures that the titration endpoint is sharp and well-defined, allowing for consistent and reliable measurements.** This precision is particularly valuable in complex reactions or when dealing with small sample sizes, where traditional methods may struggle to provide consistent results.**
In summary, back titration is a powerful technique that empowers chemists with accurate and precise results, even in challenging analytical scenarios. Its ability to overcome slow or incomplete reactions makes it a valuable tool for a wide range of chemical analysis applications.
Applications of Back Titration: Unveiling Its Versatile Utility
Back titration, a technique employed in analytical chemistry, offers a unique approach to determining the concentration of a substance. Unlike traditional titration methods, back titration involves adding an excess of a reagent to the sample and subsequently titrating the excess with a second reagent. This technique proves particularly advantageous when the analyte undergoes slow or incomplete reactions.
One of the most common applications of back titration is in acid-base titrations. This method is particularly useful when the acid or base is weak, as it provides accurate results even in the presence of incomplete reactions. Back titration is also employed in precipitation reactions, such as the determination of chloride ions using the Mohr method.
In addition to acid-base and precipitation reactions, back titration finds applications in complexometric titrations, where it is used to determine the concentration of metal ions. This technique is also utilized in redox titrations, such as the determination of iron(II) ions using potassium permanganate.
The versatility of back titration extends to biochemical assays, where it is used to determine the activity of enzymes. For instance, in the determination of amylase activity, back titration is employed to measure the amount of glucose produced by the enzyme over a specific time interval.
Furthermore, back titration has applications in food analysis, environmental monitoring, and pharmaceutical analysis. In the food industry, it is used to determine the acidity of fruits and vegetables, while in environmental monitoring, it is employed to measure the concentration of pollutants in water and soil samples. In pharmaceutical analysis, back titration is used to determine the purity and potency of active pharmaceutical ingredients.
Advantages and Disadvantages of Back Titration
In the realm of chemistry, back titration emerges as a unique and valuable technique that complements traditional titration methods. While both techniques share the common goal of determining the concentration of an unknown analyte, back titration offers distinct advantages and faces certain challenges that set it apart.
Advantages:
-
Enhanced Accuracy and Precision: Back titration boasts remarkable accuracy and precision due to its ability to overcome certain reaction limitations. By titrating the excess reagent rather than the analyte directly, this technique minimizes errors associated with slow or incomplete reactions, providing more reliable quantitative data.
-
Versatility in Application: Back titration finds its niche in various analytical scenarios, particularly when dealing with complex mixtures or when the analyte undergoes reactions that may not be suitable for direct titration. This versatility makes it a valuable tool for a wide range of applications.
Disadvantages:
-
Complexity and Time Consumption: Compared to traditional titration, back titration involves additional steps and calculations, which can add to its complexity. This increased complexity may require specialized knowledge and may extend the overall analysis time.
-
Potential for Error Accumulation: While back titration offers enhanced accuracy, it also introduces the possibility of error accumulation at each step. This potential drawback necessitates meticulous technique and careful consideration of all experimental variables to ensure reliable results.
Overall, back titration offers a powerful alternative to traditional titration methods, particularly when precision and accuracy are paramount. However, its complexity and potential for error accumulation should be carefully weighed against its advantages. With proper understanding and skilled execution, back titration remains an indispensable tool for a diverse range of chemical analyses.