Unlock The Energy-Carrying Power Of Atp: Ribose, The Pentose Sugar Backbone
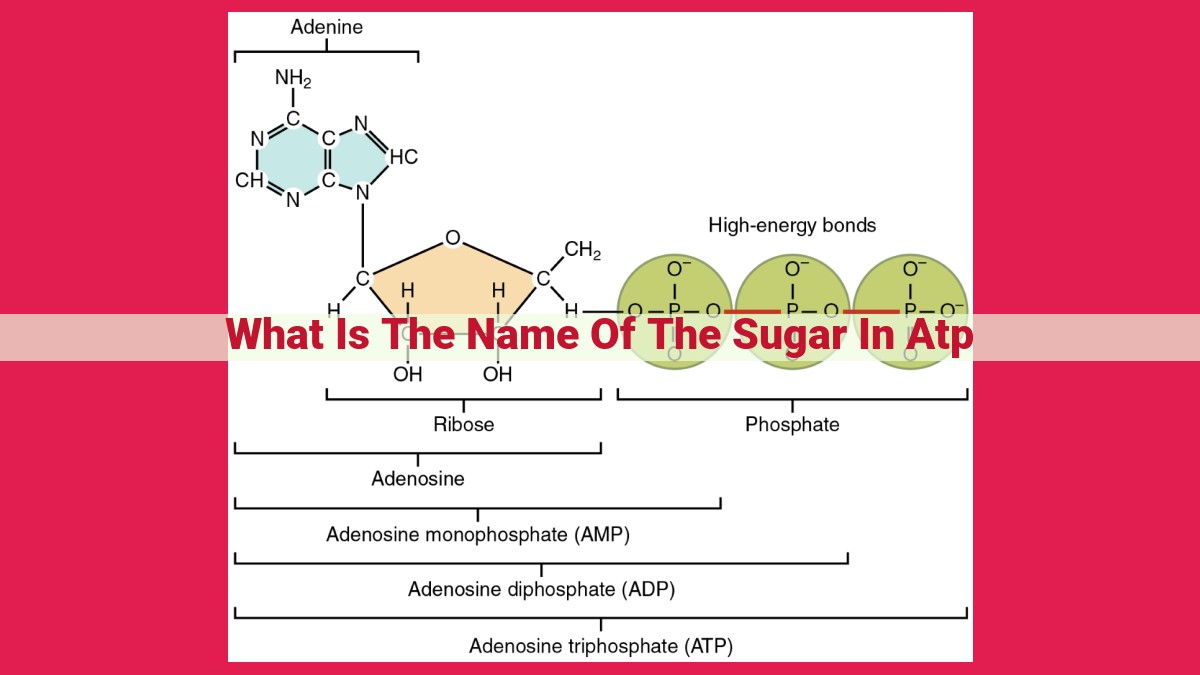
The sugar in ATP is a pentose sugar called Ribose, a molecule with five carbon atoms. Ribose forms the backbone of the ATP molecule, connecting the three phosphate groups. Its structure and properties play a critical role in ATP’s function as an energy carrier, facilitating the storage and release of energy within cells.
- Briefly introduce the purpose of the post: to identify the sugar in ATP.
The Sweet Secret: Unveiling the Sugar that Powers Our Cells
In the bustling metropolis of our bodies, there resides a tiny yet formidable molecule that holds the key to our cellular vitality: ATP. This ubiquitous energy currency fuels every aspect of life, from the beating of our hearts to the blinking of our eyelids. But what’s the secret ingredient that gives ATP its remarkable energy-storing prowess? It’s none other than ribose, the sweet-tasting sugar that forms the backbone of this essential molecule.
Just as a recipe requires carefully selected ingredients to achieve its desired flavor, the structure of ATP hinges on the presence of ribose. This pentose sugar, boasting five carbon atoms, forms the central core of the ATP molecule, its delicate bonds connecting the three phosphate groups. It’s as if ribose plays the role of a master puppeteer, coordinating the movement and release of the molecule’s energy.
Unveiling the Sweet Secret: Ribose, the Sugar in ATP
At the heart of cellular energy lies a remarkable molecule: adenosine triphosphate (ATP). This energy currency powers countless biological processes, enabling movement, growth, and chemical reactions that sustain life. But what many don’t know is the hidden sugar that forms its backbone: ribose.
Ribose: A Sugar with a Vital Role
Ribose belongs to a class of sugars known as pentoses, meaning it possesses five carbon atoms. Within the ATP molecule, ribose acts as the structural foundation, connecting the three phosphate groups that hold the key to its energy storage and release capabilities.
The Structural Blueprint of ATP
Imagine a chain with ribose as the central link. Like the vertebrae of a spine, ribose provides the backbone to which the phosphate groups attach. The result is a robust molecule that can store chemical energy in the form of high-energy phosphate bonds.
Ribose’s Unique Properties
As a monosaccharide, ribose has a simple molecular structure with the formula C5H10O5. It bears a striking resemblance to its counterpart in DNA, deoxyribose, which differs only by the absence of one oxygen atom.
The Importance of Ribose in ATP Function
Ribose’s backbone structure contributes to ATP’s remarkable energy storage and release properties. The high-energy phosphate bonds between the phosphate groups are stabilized by the ribose molecule, allowing them to accumulate potential energy. When the body needs to tap into this energy, ribose plays a crucial role in the hydrolysis reaction that releases the energy contained within the phosphate bonds.
Ribose, the sugar in ATP, is not just a mere accessory. It’s the very building block that gives ATP its structural integrity and allows it to serve as the universal energy currency of life. Without ribose, ATP would lose its ability to power the countless processes that keep us alive and functioning.
Ribose’s Role in ATP: The Energy Powerhouse of Cells
In the bustling city of our cells, ATP reigns supreme as the universal energy currency. Driving the biochemical machinery that keeps us alive, ATP stores and releases energy with remarkable efficiency. And at the heart of this energy powerhouse lies a remarkable sugar molecule—ribose.
The Backbone of ATP
ATP, short for adenosine triphosphate, is a nucleotide composed of a nitrogenous base (adenine), a ribose sugar, and a chain of three phosphate groups. Ribose, a pentose sugar with five carbon atoms, forms the backbone of the ATP molecule, connecting the adenine base to the phosphate groups.
Unlocking Energy through Ribose’s Properties
Ribose’s unique properties play a vital role in ATP’s ability to store and release energy. The ribose molecule contains a high-energy bond between the second and third phosphate groups. When this bond is broken, energy is released, driving the work of cellular processes.
The structure of ribose also contributes to ATP’s stability. The molecule’s cyclic form and the presence of hydroxyl groups on its carbon atoms allow for the formation of hydrogen bonds. These bonds create a stable molecular cage that protects the high-energy phosphate bond.
Beyond Energy Storage
Ribose’s role in ATP extends beyond energy storage and release. Its presence also governs the specificity of ATP interactions with enzymes. This specificity ensures that ATP is used only in specific biochemical reactions, preventing energy waste and maintaining cellular homeostasis.
A Cellular Workhorse
In the bustling world of our cells, ATP, fueled by the power of ribose, serves as the tireless workhorse, providing the energy that drives every aspect of cellular life. From muscle contractions to nerve impulses, from DNA synthesis to protein production, ATP is the universal language of energy, and ribose is the key that unlocks its potential.
The Sweet Secrets of ATP: Unlocking the Sugar Behind Cellular Energy
In the bustling metropolis of our cells, a remarkable molecule reigns supreme, orchestrating the intricate dance of life: ATP. This energy currency powers every cellular process, from muscle contractions to DNA synthesis. But what’s the secret ingredient that fuels ATP’s extraordinary capabilities? It’s a humble yet vital sugar called ribose.
Ribose: The Backbone of ATP
Ribose, a pentose sugar with five carbon atoms, forms the backbone of the ATP molecule. It interconnects the three phosphate groups like a sturdy bridge, providing a stable structure that holds the molecule together. This arrangement is crucial for ATP’s energy-storing and releasing properties.
The Dance of Phosphate Groups
When a cell needs energy, enzymes hydrolyze the bond between the second and third phosphate groups in ATP. This releases energy that powers cellular activities. Ribose’s unique structure allows for this precise and controlled energy release. Its hydroxyl groups form hydrogen bonds with the phosphate groups, stabilizing them and ensuring that energy is released steadily.
Ribose: A Monosaccharide with a Familiar Cousin
Ribose belongs to the monosaccharide family, the simplest form of carbohydrates. It has a structural formula of C5H10O5. Interestingly, ribose shares a structural similarity with deoxyribose, the sugar found in DNA. Both sugars have a five-carbon ring structure but differ in their presence or absence of an oxygen atom on the second carbon atom.
Ribose, the sugar in ATP, plays an indispensable role in cellular energy metabolism. Its unique structure enables the controlled release of energy from ATP, powering the myriad processes that sustain life. Understanding the relationship between ribose and ATP deepens our appreciation for the intricate workings of our cells and the vital role of carbohydrates in fueling our existence.