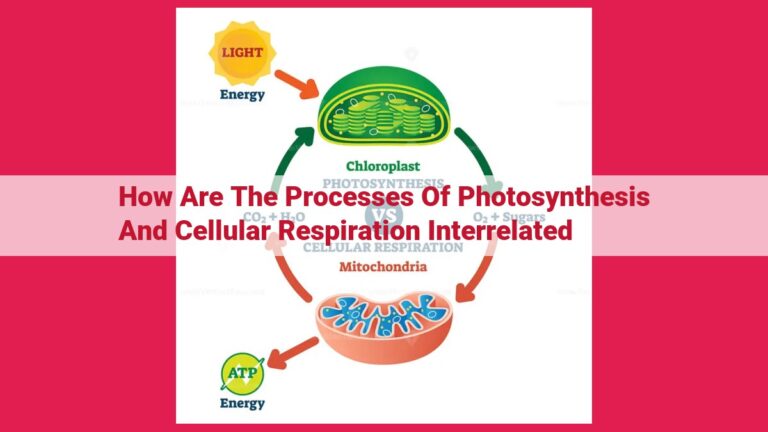
Unveiling The Atp Molecule: The Powerhouse Of Cellular Energy
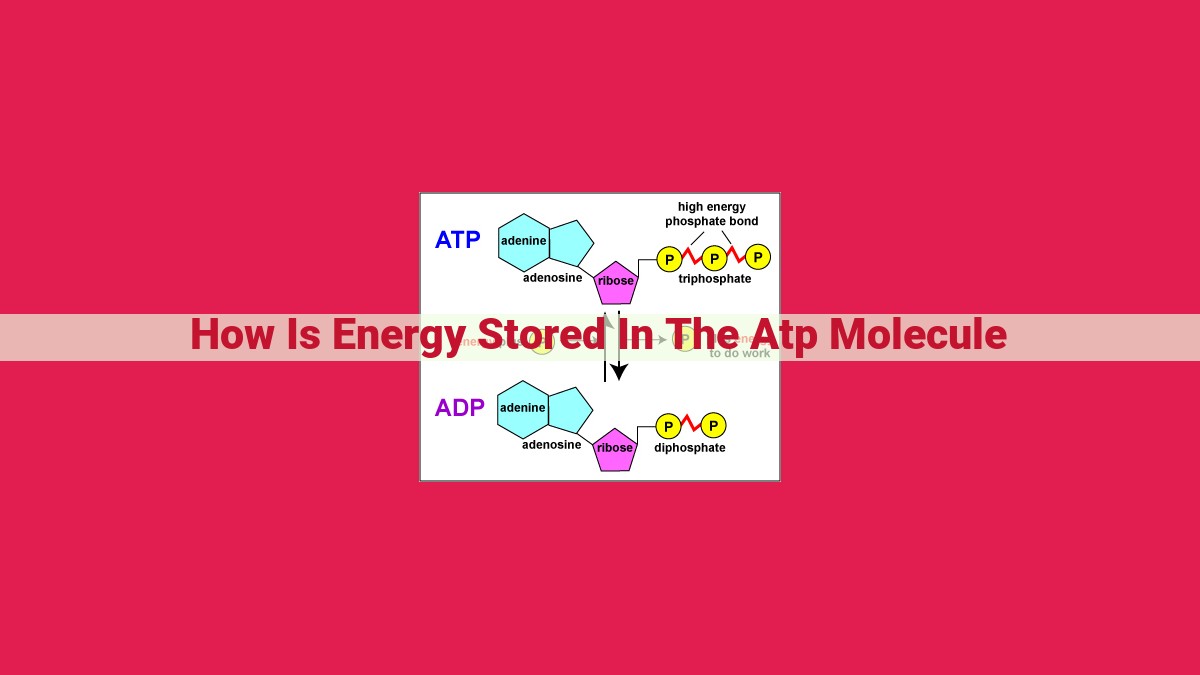
Energy is stored in the ATP molecule through high-energy phosphoanhydride bonds that connect the three phosphate groups. When these bonds are broken through hydrolysis, a significant amount of energy is released. This energy can be utilized to power various cellular processes, making ATP the primary energy currency in living organisms. Its ability to diffuse through cell membranes and act as a universal energy carrier allows cells to efficiently transfer and utilize energy for essential functions.
Phosphoanhydride Bonds: The Energy Reservoirs
- Explain how phosphoanhydride bonds connect the phosphate groups in ATP and store energy.
Unveiling the Energy Secrets of Phosphoanhydride Bonds: The Fuel Behind Life’s Processes
In the realm of biochemistry, energy takes center stage, driving countless cellular processes that sustain life. At the heart of this energy circuitry lies a remarkable molecule called adenosine triphosphate (ATP), the “universal energy currency” of living organisms. What makes ATP so extraordinary is its ability to store and release energy through its unique phosphoanhydride bonds.
The Power of Phosphoanhydride Bonds
Phosphoanhydride bonds are high-energy chemical linkages that connect the phosphate groups in ATP. These bonds resemble tightly coiled springs, containing a vast reservoir of energy that can be harnessed for cellular activities. The strength of these bonds ensures that the stored energy remains stable until needed.
Unlocking Energy through Hydrolysis
When the cell requires energy, the phosphoanhydride bonds in ATP undergo a process called hydrolysis. During hydrolysis, a water molecule breaks the bond between the terminal phosphate group and the rest of the molecule. This breaking of the bond releases a significant amount of energy, which can then be used to power cellular machinery.
ATP: A Versatile Energy Courier
ATP’s compact structure and solubility allow it to diffuse through cell membranes, making it an ideal energy courier. It can travel throughout the cell, delivering energy to wherever it is needed. The phosphate groups in ATP serve as the “handles” that enzymes can grab onto, facilitating the transfer of energy to specific cellular processes.
ATP: The Universal Energy Currency
ATP plays a crucial role in energy transfer between cells. As the “universal energy currency,” it serves as a common denominator for energy exchange, ensuring that different metabolic pathways can seamlessly communicate. This energy conversion system enables cells to respond to changing energy demands and maintain homeostasis.
In conclusion, phosphoanhydride bonds are the linchpin of ATP’s energy-storage capabilities. Hydrolysis of these bonds releases energy that fuels countless cellular processes. ATP, with its versatility as an energy courier and its universal acceptance as the energy currency, ensures that life’s essential activities can proceed seamlessly. The understanding of phosphoanhydride bonds and ATP’s role is a testament to the intricate dance of energy that underpins the very fabric of life.
Hydrolysis of Phosphoanhydride Bonds: Unlocking the Energy Within
In the realm of cellular energy, phosphoanhydride bonds stand as the guardians of a hidden energy reserve. These intricate molecular structures, found within the powerhouse molecule ATP, serve as the key to unlocking the power that drives countless biological processes.
The Process of Hydrolysis: Breaking Bonds, Releasing Energy
Hydrolysis, a fundamental chemical reaction, plays a crucial role in the release of this stored energy. Imagine ATP as a coiled spring, with the phosphoanhydride bond acting as the tension keeping it compressed. When the hydroxyl group of water attacks the bond, it cleaves in two, releasing a burst of energy.
This energy is harnessed by cells to fuel a multitude of essential functions. From muscle contraction to nerve signaling, the hydrolysis of ATP provides the driving force for countless biological reactions.
The Energetic Implications: A Chain Reaction
The release of energy from phosphoanhydride bond hydrolysis is not an isolated event. It initiates a chain reaction, cascading through cellular processes. ATP serves as a versatile energy courier, shuttling energy from one reaction to another.
As ATP donates its energy through hydrolysis, it transforms into ADP (adenosine diphosphate), a molecule with a lower energy content. However, this ADP is quickly replenished by cellular processes, ensuring a continuous supply of energy-rich ATP.
Optimized for SEO on Page
- Keywords: phosphoanhydride bonds, hydrolysis, ATP, energy, energy currency
- Heading: The Hydrolysis of Phosphoanhydride Bonds: Unlocking the Energy Source of Cells
- Meta Description: Discover the secrets of phosphoanhydride bonds, the energy reservoirs within ATP, and how hydrolysis releases the power that drives cellular processes.
ATP: The Versatile Energy Courier of Life
In the bustling metropolis of our cells, where countless biochemical processes unfold, there exists an unsung hero—Adenosine triphosphate (ATP). This remarkable molecule, known as the universal energy currency, plays a pivotal role in powering the intricate machinery of life, akin to an indefatigable postman delivering energy packets to every nook and cranny of the cell.
ATP’s remarkable versatility stems from its unique structure and properties. Its intricate molecular architecture features a backbone of three phosphate groups connected by high-energy phosphoanhydride bonds. These bonds are the key to ATP’s energetic prowess, storing a vast reserve of chemical energy awaiting release.
When the cell demands energy, ATP undergoes hydrolysis—the process of breaking down those high-energy bonds. As the terminal phosphate group is cleaved, liberating energy, it is transferred to other molecules, fueling the countless reactions that drive cellular life. Think of ATP as a generous patron, donating its precious energy to enable essential cellular functions such as muscle contraction, nerve impulse transmission, and the synthesis of new molecules.
The beauty of ATP lies not only in its ability to power cellular processes but also in its remarkable mobility. Unlike other energy-rich molecules, ATP can permeate cell membranes, the boundaries that separate different compartments within the cell. This unique ability is crucial for ATP to reach all corners of the cell, analogous to a skilled courier navigating through a complex maze, delivering energy parcels to every destination in need.
In conclusion, ATP stands as the cornerstone of cellular energy management. Its structure, properties, and mobility allow it to fulfill its vital role as the universal energy currency, ensuring that the symphony of life continues uninterrupted within every cell of our bodies.
ATP: The Universal Energy Currency That Powers Life’s Processes
In the bustling streets of our cities, we witness a constant flow of energy, from the revving engines of cars to the bright lights illuminating the night sky. But deep within the microscopic realm of cells, a different kind of energy exchange is taking place, one that is essential for the very survival of life. This energy currency, known as ATP, is the universal fuel that powers a myriad of cellular processes, from muscle contraction to nerve impulse transmission.
ATP stands for adenosine triphosphate. It is a small molecule that consists of an adenine base, a ribose sugar, and three phosphate groups. The key to ATP’s energetic prowess lies in the phosphoanhydride bonds that connect its phosphate groups. These bonds are high-energy linkages that store a significant amount of chemical energy. When these bonds are broken, the energy is released, providing the power to drive cellular reactions.
The process of hydrolysis breaks down ATP by cleaving off one of its phosphate groups. This hydrolysis reaction is coupled to various cellular processes, allowing the energy released from the bond to be harnessed for specific tasks. Just as a hydroelectric dam harnesses the energy of flowing water to generate electricity, cells utilize the hydrolysis of ATP to drive energy-requiring reactions.
The versatility of ATP as an energy carrier stems from its ability to diffuse freely through cell membranes. This enables ATP to reach all corners of the cell, delivering energy to organelles and structures where it is needed. Moreover, ATP acts as a common currency for energy exchange between different biochemical pathways. By converting the energy of one reaction into ATP, cells can make it available for use in other reactions, ensuring a seamless flow of energy throughout the cell.
In summary, ATP is the universal energy currency of all living organisms. Its phosphoanhydride bonds store a vast reservoir of chemical energy, which is released upon hydrolysis to power a myriad of cellular processes and facilitate the transfer of energy between different biochemical pathways. ATP’s versatility and efficiency make it the cornerstone of life, enabling cells to function and thrive in the dynamic and energy-demanding environment of living organisms.