Atoms, Elements, And Compounds: The Building Blocks Of Matter
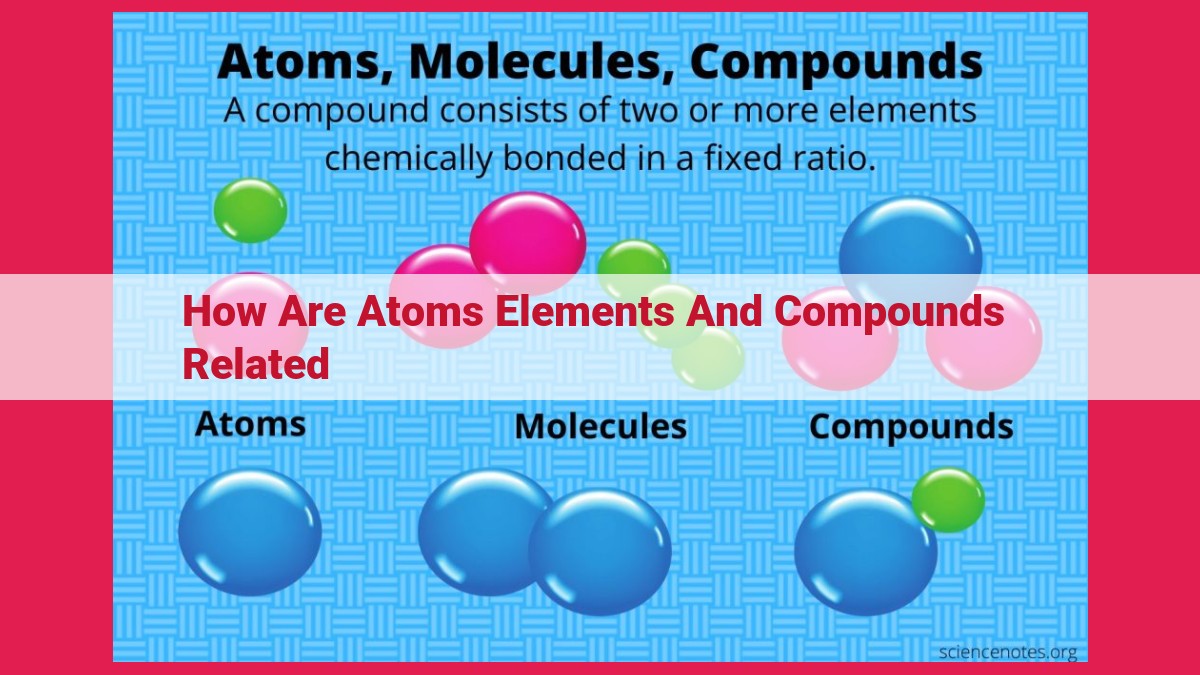
Atoms are the fundamental building blocks of matter, composed of negatively charged electrons around a positively charged nucleus. Elements are pure substances that contain atoms of only one type, while compounds are formed when atoms of different elements chemically bond. Elements are represented by their chemical symbols, and compounds are described by chemical formulas that indicate the composition of elements present and their relative proportions.
Unraveling the Enigma of Matter: A Journey into the Basic Building Blocks
As we embark on a captivating odyssey into the realm of chemistry, let’s begin at the very foundation: matter. Matter, the essence of everything around us, comprises atoms, the indivisible fundamental units that define the nature of every substance.
Each atom is composed of a nucleus and electrons. The nucleus, the heart of the atom, contains protons and neutrons, while the electrons orbit the nucleus, occupying distinct energy levels. Protons possess a positive charge, neutrons are neutral, and electrons carry a negative charge. The interplay of these subatomic particles establishes the atom’s behavior and properties.
Atoms of the same element share an identical number of protons in their nuclei. Elements are the basic building blocks of matter, each possessing a unique set of protons and electrons. They form the foundation of the periodic table, a systematic arrangement of elements based on their atomic numbers, which dictate the number of protons in each atom. The periodic table reveals patterns in chemical properties that guide our understanding of matter’s composition and behavior.
Unlocking the Secrets of Chemical Bonding
Imagine a world where all matter existed as separate and non-reactive atoms, like isolated stars in a vast cosmic expanse. Life as we know it would cease to exist. But thankfully, nature has a secret weapon: chemical bonding.
Chemical bonding is the glue that holds atoms together, forming the building blocks of everything around us. It’s the force that transforms inert elements into functional molecules, enabling reactions that drive life and shape our world.
Types and Mechanisms of Chemical Bonding
There are various types of chemical bonding, each with its unique mechanism and characteristics:
- Covalent Bonding: Imagine atoms as magnets, attracting and sharing electrons to form stable pairs. This type of bonding is found in molecules like water (H2O) and methane (CH4).
- Ionic Bonding: Two atoms exchange their electrons like a cosmic game of hot potato, resulting in oppositely charged ions. This bonding forms compounds such as salt (NaCl).
- Metallic Bonding: Metals share a “sea” of electrons, creating a conductive and malleable material. This type of bonding is responsible for the strength and ductility of metals.
Understanding these bonding mechanisms is crucial for unraveling the complexities of the molecular world. It’s the key to uncovering the properties, reactions, and applications of countless chemical substances.
Chemical Formulas: Describing Compounds
- Notation and significance of chemical formulas
- Molecular formulas vs. empirical formulas
Chemical Formulas: Deciphering the Language of Compounds
In the grand tapestry of chemistry, chemical formulas play a pivotal role in describing the composition and structure of compounds. These seemingly simple strings of characters hold a wealth of information, allowing scientists to decode the building blocks of matter and predict their behavior.
Notation and Significance of Chemical Formulas
Chemical formulas are abbreviated representations of compounds, using symbols from the periodic table. Each symbol represents an element, which is a pure substance that cannot be broken down into simpler substances. For instance, the formula for sodium chloride is NaCl, indicating that it contains sodium (Na) and chlorine (Cl) in a 1:1 ratio.
Molecular Formulas vs. Empirical Formulas
Two types of chemical formulas are commonly used:
-
Molecular formulas provide the exact number of each type of atom in a molecule. A molecule is a specific arrangement of atoms that has a defined structure. For example, the molecular formula for glucose is C6H12O6, indicating that it consists of six carbon (C) atoms, twelve hydrogen (H) atoms, and six oxygen (O) atoms.
-
Empirical formulas show the simplest whole-number ratio of atoms in a compound. They do not provide information about the molecule’s structure. For instance, the empirical formula for glucose is CH2O, which means that it contains carbon, hydrogen, and oxygen in a 1:2:1 ratio.
Unveiling the Molecular Architecture: Structural Formulas
Structural formulas are visual representations of molecules that depict the arrangement of atoms and the bonds between them. These formulas use various symbols and lines to represent atoms, bonds, and molecular shape. By examining a structural formula, scientists can gain insights into the compound’s properties and reactivity.
Determining Molecular Formulas
Determining the molecular formula of a compound requires knowing the mass of each element present and their atomic weights. The empirical formula can be used as a starting point, along with the molecular weight, to determine the actual number of atoms in the molecule.
Applications of Empirical Formulas
Empirical formulas are invaluable for determining the chemical composition of compounds, particularly when molecular weight data is not available. They also allow scientists to compare substances with similar molecular ratios, revealing patterns and relationships within chemical series.
**Structural Formulas: Unveiling the Intricate Architecture of Molecules**
In the realm of chemistry, unraveling the secrets of matter requires understanding not only its basic building blocks but also the intricate connections that hold them together. Structural formulas emerge as a powerful tool in this endeavor, offering a visual representation of molecular architecture that grants us a window into the very essence of chemical substances.
Visualizing Molecular Structures:
Imagine a beautiful tapestry woven with threads of different colors, each thread representing an atom. Structural formulas present a similar visual tapestry, depicting the arrangement of atoms within a molecule. By connecting these atomic symbols with lines or dots, we reveal the molecular framework, akin to an architect’s blueprint.
Unveiling Bonding Patterns:
Beyond visualizing atomic arrangement, structural formulas also portray the chemical bonds that unite atoms into a cohesive whole. These bonds, represented by lines of varying thickness, reflect the strength and nature of the interactions between atoms. Single lines indicate single bonds, double lines represent double bonds, and so on.
Shape Matters:
The spatial arrangement of atoms, depicted by structural formulas, not only influences molecular shape but also profoundly impacts the molecule’s properties and behavior. For instance, linear molecules have a straight-chain structure, while branched molecules exhibit more intricate shapes. These variations in shape can affect everything from reactivity to solubility.
In Summary:
Structural formulas are invaluable tools for understanding the molecular world, providing a visual representation of atomic arrangements and chemical bonding. They allow us to comprehend the intricate architecture of substances, predict their properties, and ultimately unravel the secrets of their behavior. Whether you’re a seasoned chemist or embarking on your scientific journey, embracing the power of structural formulas will empower you to navigate the molecular landscape with confidence and insight.
Determining Molecular Formulas: Unraveling the Structure of Compounds
In the tapestry of chemical knowledge, determining molecular formulas is an essential task, enabling us to decipher the precise arrangement of atoms within molecules. This intricate process lies at the heart of understanding the properties and behavior of countless substances that shape our world.
Empirical Formulas: A Primer
Before delving into molecular formulas, let’s revisit empirical formulas. These simplified expressions represent the smallest whole-number ratio of elements present in a compound. They provide a basic framework for understanding the overall composition of a substance. However, empirical formulas fall short in revealing the exact number of atoms of each element within the molecule.
From Empirical to Molecular Formulas
To bridge this gap, scientists employ molecular formulas. Unlike empirical formulas, molecular formulas specify the precise number of atoms of each element in a molecule. Determining molecular formulas requires two crucial pieces of information:
- Empirical formula
- Molecular weight
The molecular weight is the sum of the atomic weights of all the atoms in a molecule. By comparing the molecular weight to the empirical formula, we can determine the actual number of each type of atom present.
A Step-by-Step Guide
The process of determining molecular formulas involves a series of logical steps:
- Calculate the empirical formula weight, which is the sum of the atomic weights of all the atoms in the empirical formula.
- Divide the molecular weight by the empirical formula weight.
- Multiply the subscripts in the empirical formula by the result from step 2.
The resulting formula represents the molecular formula of the compound.
Applications in the Realm of Chemistry
Molecular formulas play a pivotal role in various chemical endeavors:
- Determining Chemical Composition: By analyzing molecular formulas, scientists can determine the exact chemical composition of substances. This knowledge is essential for characterizing the properties of materials and predicting their reactivity.
- Comparison of Compounds: Molecular formulas allow for the comparison of different compounds with similar empirical formulas. By comparing the number of atoms of each element, chemists can gain insights into the molecular structures and properties of these compounds.
In essence, determining molecular formulas is a fundamental skill in chemistry, providing a gateway to comprehending the intricate world of molecular structure and its implications in countless chemical processes.
Unveiling the Practical Uses of Empirical Formulas
Understanding Empirical Formulas
Empirical formulas, simplistic representations of compounds, reveal the ratios of elements present in them. While they don’t depict the exact molecular structure, they provide valuable insights into a compound’s chemical composition.
Applications of Empirical Formulas
1. Determining Chemical Composition:
Empirical formulas serve as analytical tools to determine the chemical composition of unknown substances. By measuring the mass of each element present in a sample, chemists can deduce its empirical formula, which provides a snapshot of its chemical makeup.
2. Comparing Substances with Similar Molecular Ratios:
Empirical formulas also allow for the comparative analysis of substances with similar molecular ratios. By comparing the empirical formulas of different compounds, scientists can identify common chemical motifs and infer their potential relationships and functionalities.
Real-World Examples
- Combustion Analysis: Empirical formulas are crucial in combustion analysis, where the masses of carbon, hydrogen, and oxygen in a compound are measured to determine its empirical formula and identify its potential fuel properties.
- Pharmaceutical Research: In drug development, empirical formulas help identify the molecular structure and chemical properties of potential drug candidates, influencing their therapeutic efficacy and safety profiles.
- Environmental Monitoring: Empirical formulas are used to analyze the composition of environmental samples, such as air and water pollution levels, providing insights into the presence and potential risks of various contaminants.
Empirical formulas, though simplistic in nature, provide powerful insights into the chemical composition of substances. They empower scientists to solve analytical challenges, understand molecular relationships, and make informed decisions in various fields of science and industry. By unveiling the practical applications of empirical formulas, we gain a deeper appreciation for their crucial role in shaping our understanding of the chemical world.