Atomic Structure: Unraveling The Distinctive Characteristics Of Elements
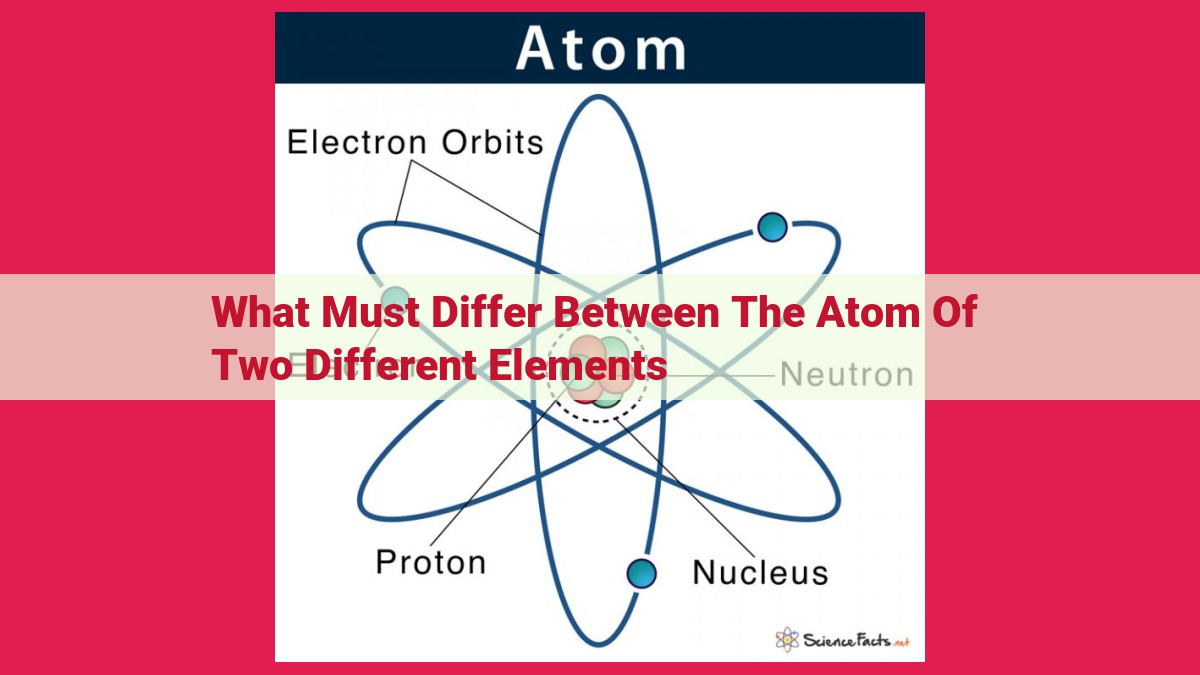
The atoms of different elements differ in three key aspects: the number of protons, the number of neutrons, and the number of electrons. The number of protons determines the element’s identity and is unique for each element. The number of neutrons affects the isotope of the element and influences its mass. Finally, the number of electrons plays a crucial role in chemical properties and behavior.
Atomic Fundamentals
- Define an atom and its components (protons, neutrons, electrons)
Unlocking the Secrets of Atoms: A Journey into the Heart of Matter
Atoms, the fundamental building blocks of everything in our universe, hold countless mysteries that have captivated scientists for centuries. Let’s embark on an atomic adventure to unveil some of their secrets, starting with the very basics.
Meet the Atom: Protons, Neutrons, and Electrons
An atom is the smallest unit of matter that retains the chemical properties of an element. It consists of a positively charged nucleus surrounded by a cloud of negatively charged electrons. The nucleus contains two types of particles: protons and neutrons. Protons carry a positive charge and determine the number of electrons an atom can have, establishing its atomic number. Neutrons have no charge and serve as the glue that holds protons together. Electrons orbit the nucleus at specific energy levels, influencing an atom’s chemical behavior.
Proton Power: Defining Atomic Identity
At the heart of every atom lies a tiny, positively charged nucleus, which holds the key to the atom’s very existence. Within this nucleus reside protons, the fundamental particles that determine an atom’s identity.
The number of protons in an atom’s nucleus defines its atomic number. This number is unique for each element, like a fingerprint that distinguishes one element from another. For instance, all atoms with one proton are hydrogen atoms, while those with two protons are helium atoms.
The atomic number also dictates the atomic structure of an atom. It determines the number of electrons that orbit the nucleus, as atoms strive to maintain a balanced electrical charge. The number of electrons, in turn, influences the chemical properties of the element, shaping how it interacts with other atoms.
Nuclear physics delves into the realm of the nucleus, where protons play a pivotal role. The number of protons in a nucleus affects the strong nuclear force, which holds the nucleus together against the repulsive electrostatic forces between protons. This delicate balance influences the stability of atoms and their susceptibility to radioactive decay.
In conclusion, protons, with their unique number and charge, define the very essence of atomic identity. They not only determine the atomic number but also shape the atomic structure and influence chemical properties. Understanding the power of protons is essential for unraveling the mysteries of the atomic world.
Neutron Nuances: Isotopes and Beyond
Understanding the Core: Isotopes and Their Significance
Apart from protons and electrons, another fundamental component of an atom is the neutron. Neutrons reside in the atomic nucleus alongside protons, contributing to the atom’s overall mass. However, unlike protons, neutrons possess no electrical charge, giving them a neutral character.
Neutron Variability: The Origin of Isotopes
Within an element, the number of protons remains constant, defining its atomic identity. However, the number of neutrons can vary, leading to the formation of isotopes. Isotopes are atoms of the same element with identical numbers of protons but differing numbers of neutrons. For instance, carbon exists as carbon-12, carbon-13, and carbon-14, each with six protons but varying neutron numbers.
Isotopes in Nuclear Physics: Unraveling the Atomic Mass
The presence of different isotopes significantly impacts the atomic mass of an element. Atomic mass is the weighted average of the masses of all isotopes, considering their relative abundance. For example, chlorine has two naturally occurring isotopes, chlorine-35 and chlorine-37. Chlorine-35, with 17 protons and 18 neutrons, is more abundant than chlorine-37, containing 17 protons and 20 neutrons. Consequently, the atomic mass of chlorine is closer to that of chlorine-35.
Neutron Influence on Radioactivity: Unstable Nuclei
Neutrons play a crucial role in nuclear stability. When the neutron-to-proton ratio in an atomic nucleus is imbalanced, the nucleus can become radioactive. Radioactive isotopes decay over time, emitting particles or energy to achieve a more stable configuration. Carbon-14, a radioactive isotope of carbon, undergoes beta decay, releasing an electron and transforming into nitrogen-14. This decay process is widely utilized in various fields, including dating ancient artifacts and tracing biological processes.
The varying neutron numbers within elements give rise to isotopes, which contribute to an element’s unique properties. Understanding the role of isotopes not only enhances our knowledge of atomic structure but also has significant implications in nuclear physics, chemistry, and other scientific disciplines.
**Electrons: Shaping Chemical Behavior**
In the intricate world of matter, electrons play a crucial role in shaping the very essence of elements. They are the tiny, negatively charged particles that orbit the nucleus of an atom, and their number profoundly influences the chemical properties that define each element.
The outermost electrons, known as valence electrons, hold the key to chemical bonding. These electrons determine an element’s ability to interact with other atoms, forming molecules and compounds. Elements with a high number of valence electrons tend to be more reactive, readily forming bonds to achieve a stable configuration. Conversely, elements with fewer valence electrons are less reactive and may exhibit more inert behavior.
The number of valence electrons is directly related to an element’s position on the periodic table. Elements in the same group (vertical column) have the same number of valence electrons and therefore share similar chemical properties. For instance, all alkali metals have one valence electron, giving them a distinctive reactivity.
The interplay between electron number and chemical bonding is evident in the formation of ionic and covalent bonds. Ionic bonds occur when an atom loses or gains electrons to achieve a stable electron configuration, creating charged ions that attract each other electrostatically. Covalent bonds, on the other hand, involve the sharing of electron pairs between atoms, resulting in molecules held together by mutual attraction.
Understanding electron number and its impact on chemical behavior is crucial in various scientific fields. It guides the design of new materials, helps explain the reactivity of biological molecules, and even plays a role in understanding the chemical reactions that occur in stars. By unraveling the secrets of electrons, we gain deeper insights into the fundamental forces that govern the world around us.
Electron Arrangement: Beyond Numbers
The intricate dance of electrons within an atom goes beyond mere numbers. It’s a captivating choreography that orchestrates the structure and behavior of matter at its most fundamental level. As we delve into this mesmerizing realm, we’ll unravel the profound influence of electron arrangement on the shape of molecules and their remarkable properties.
Atomic Orbitals: The Electron’s Home
Electrons reside within designated regions surrounding the atom’s nucleus, known as atomic orbitals. These orbitals are like cozy apartments, each with a characteristic shape and energy level. Electrons occupy these orbitals in pairs, spinning in opposite directions like spinning tops.
Shells: Layered Electron Neighborhoods
Atomic orbitals are organized into layers called electron shells. Each shell has a specific energy level and can accommodate a certain number of electrons. The first shell, closest to the nucleus, can hold up to two electrons, while subsequent shells have increasing capacities.
Molecular Geometry: Electron Dance and Molecule Shape
The arrangement of electrons in orbitals directly influences the geometry of molecules. The shape of a molecule is determined by the number of electron pairs in its outermost shell and the manner in which these pairs repel each other. Linear molecules, like carbon dioxide (CO2), have electron pairs arranged in a straight line, while molecules with bent shapes, such as water (H2O), have electron pairs that push against each other, bending the molecule.
Physical Properties: Electrons Shape Matter
The electron arrangement in an atom not only affects its molecular shape but also governs its physical properties. Elements with similar electron arrangements exhibit similar physical properties. For instance, noble gases have their outermost electron shell completely filled, making them highly stable and unreactive. Metals have loosely bound electrons in their outermost shell, granting them high electrical and thermal conductivity.
The arrangement of electrons within an atom is a crucial factor that differentiates one element from another. It determines the shape and properties of molecules, shaping the behavior of matter on a macroscopic scale. This intricate ballet of electrons is a testament to the extraordinary complexity and beauty found at the very heart of our universe.
Mass Number: The Whole Picture
The Essence of an Atom’s Identity
In the realm of atomic science, each element possesses a distinctive mass number, a fundamental characteristic that sets it apart from its atomic counterparts. This enigmatic number, denoted by the symbol A, represents the total number of protons and neutrons cuddled within an atom’s core, the nucleus.
Delving into Proton and Neutron Counts
Protons, with their positive charge, wield immense power in defining an element’s identity. Their number, known as the atomic number, determines an element’s position on the periodic table, dictating its place among the chemical family tree. Conversely, neutrons, lacking an electrical charge, play a vital role in an atom’s stability and mass.
The Dance of Isotopes
Atoms of the same element can come in different isotopic forms, each with a unique neutron count. These isotopic variations arise from fluctuations in the neutron population, giving birth to isotopes with identical atomic numbers but distinct mass numbers.
Mass Number Unveiled
The mass number seamlessly marries the proton count and neutron count, providing a numeric snapshot of the atom’s nucleus. This number holds great significance in nuclear physics, shaping the nuclear mass of the atom. Isotopes, with their varying neutron counts, exert a subtle influence on their respective elemental mass numbers.
Unlocking Chemical and Physical Secrets
Mass number plays a crucial role in deciphering an element’s chemical and physical characteristics. By understanding the intricate relationship between mass number and atomic structure, scientists can unravel the mysteries that govern chemical reactions, predict physical properties, and explore the very essence of matter that makes up our world.