Unveiling The Significance Of Atomic Number: Silver’s Position And Properties
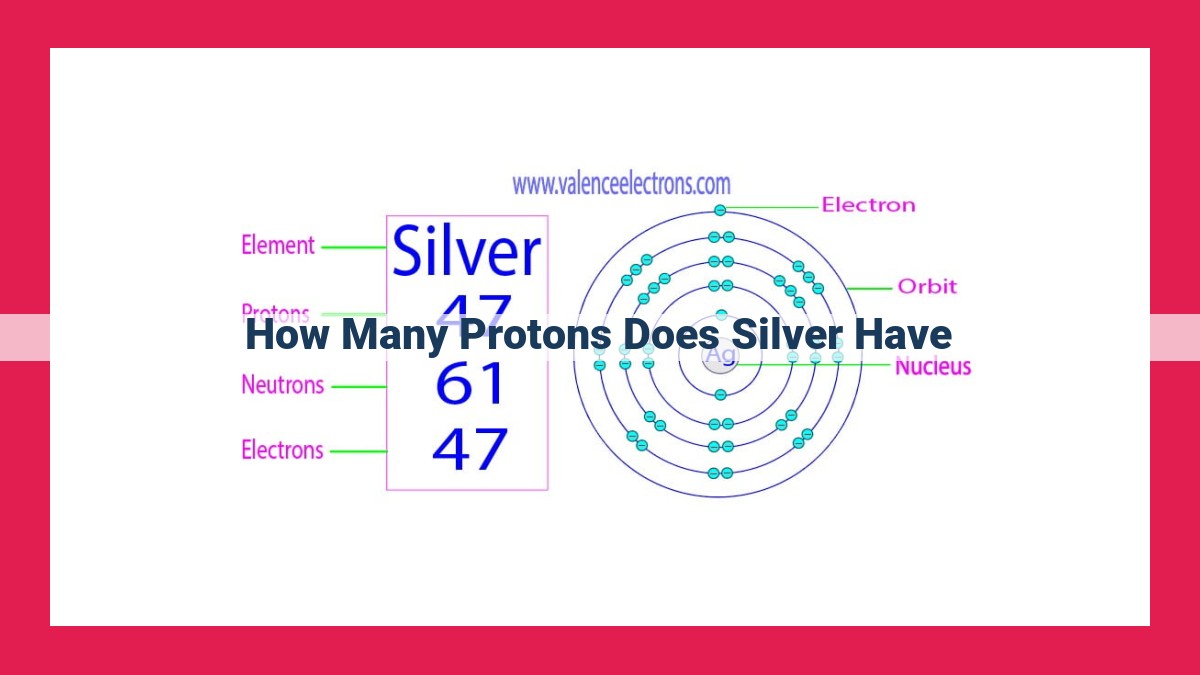
- Silver has 47 protons, its atomic number, which defines its identity as an element and its position on the periodic table. Protons are subatomic particles with a positive charge located in the nucleus, and their number determines an element’s atomic number. The atomic number governs the number of electrons in an atom, which in turn influences the atom’s chemical properties and behavior.
Unraveling the Secrets of Silver: A Journey into Atomic Number and Protons
In the world of chemistry, each element possesses a unique identity defined by its atomic number, a fundamental property that plays a pivotal role in determining its characteristics and behavior. For silver, a precious metal with a lustrous shine, this atomic number holds the key to understanding its very essence.
Atomic number is the defining trait of an element, a numerical value that corresponds to the number of protons residing within the nucleus of an atom. Protons, the positively charged subatomic particles, are the building blocks of an atom’s identity and determine its position on the periodic table, the map of all known elements.
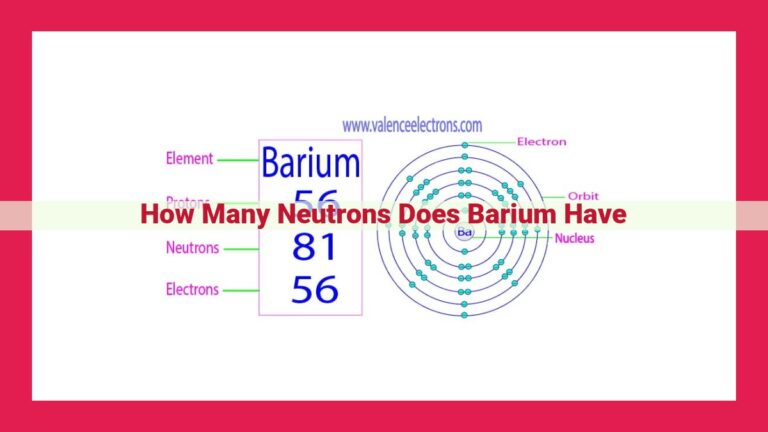
Silver, with its atomic number of 47, proudly occupies its place on the periodic table. This number signifies that each silver atom contains 47 protons, a unique fingerprint that distinguishes it from all other elements.
Atomic Number and Protons: Unveiling the Essence of Silver
In the intricate tapestry of the atomic world, each element possesses a unique identity defined by its atomic number. This fundamental property not only determines an element’s behavior but also holds the key to understanding the very building blocks of matter. In this exploration, we embark on a journey to unravel the secrets of the atomic number and uncover the fascinating story of protons, the microscopic guardians of an element’s identity.
The Atomic Number: A Defining Characteristic
At the heart of every atom lies the atomic nucleus, a dense core positively charged by protons. The number of protons within this nucleus is what defines an element’s atomic number. Each element has a unique atomic number, akin to a fingerprint, that distinguishes it from all others.
Silver: A Noble Metal with an Atomic Number of 47
Silver, a precious metal renowned for its lustrous sheen, resides in Group 11 of the periodic table. Its atomic number is 47, indicating that every silver atom contains 47 protons. This fundamental characteristic places silver below palladium (atomic number 46) and above cadmium (atomic number 48).
The Periodic Table: A Map of Atomic Numbers
The periodic table serves as a visual representation of the elements, arranged in order of increasing atomic number. This arrangement highlights their similarities and differences, allowing scientists to predict their properties and behavior. Silver, with its atomic number of 47, occupies a specific position in this intricate table, reflecting its unique combination of characteristics.
Understanding Atomic Number: A Journey to Determine the Number of Protons in Silver
In the realm of chemistry, the atomic number plays a pivotal role in shaping the identity and behavior of elements. It’s like a celestial blueprint that tells us about an element’s fundamental makeup. But what exactly is the atomic number, and how can we use it to uncover the secrets of an element like silver?
Let’s embark on an atomic adventure as we explore the concept of atomic number and its significance in understanding the number of protons within silver.
Atomic Number: The Proton Count
Atomic number is an essential property that distinguishes one element from another. It represents the number of protons residing in an atom’s nucleus. Protons are subatomic particles that carry a positive charge, and their number directly determines an element’s unique identity and its position on the periodic table.
Silver’s Atomic Number: A Window into Its Identity
Silver, a gleaming precious metal, has an atomic number of 47. This means that every silver atom contains 47 protons in its nucleus. This atomic number places silver on the fifth row of the periodic table, in Group 11, among other transition metals.
Related Concepts: Unveiling the Atomic Puzzle
To gain a comprehensive understanding of atomic number, we need to explore some related concepts:
-
Element: An element is the most basic form of matter that can’t be chemically broken down any further. Each element is defined by its unique atomic number.
-
Atomic Mass: Atomic mass represents the combined mass of an atom’s protons and neutrons. It’s closely related to the atomic number, as the number of protons significantly contributes to the atomic mass.
-
Isotopes: Atoms of the same element can have different atomic masses. These variants are called isotopes. They share the same atomic number, but differ in the number of neutrons in their nuclei.
Protons: The Nucleus’s Heartbeat
Protons are the positively charged particles that reside in an atom’s nucleus. Each proton carries a charge of +1. They’re responsible for the element’s atomic number and its place in the periodic table.
The number of protons in an atom is a defining characteristic, shaping its identity and chemical reactivity. It’s like the atomic fingerprint of an element. In the case of silver, its 47 protons define its unique properties, making it the lustrous metal that we know and value.
By understanding the concept of atomic number, we gain a deeper appreciation for the fundamental building blocks of matter. It’s a powerful tool that allows us to unravel the secrets of elements and explore the fascinating world of chemistry.
Nuclear Charge: The Balancing Act of Atoms
In the heart of every atom lies a tiny universe, a nucleus teeming with subatomic particles. One of the most crucial components of this nuclear realm is the proton, a positively charged particle that plays a pivotal role in determining an element’s identity and behavior.
The total number of protons within an atom’s nucleus is known as its nuclear charge. This charge holds great significance because it directly influences the atom’s ability to attract electrons, the negatively charged particles that orbit the nucleus.
Protons possess a fundamental positive charge, a property that attracts electrons, which carry an equal and opposite negative charge. This attraction creates an electrostatic force that binds electrons to the atom, forming a stable and electrically neutral entity. Without this delicate balance between proton and electron charges, atoms would exist in a chaotic state of unbalanced electrical forces.
The nuclear charge is a defining characteristic of each element. It determines the element’s position on the periodic table and dictates its chemical behavior. Elements with different nuclear charges are distinctly different entities with unique properties. For instance, silver has a nuclear charge of 47, while gold has a nuclear charge of 79. This difference in nuclear charge accounts for their distinct chemical and physical properties.
The nuclear charge is a fundamental property that governs the behavior of atoms. It determines the atom’s ability to form bonds with other atoms and influences its reactivity and other characteristics. Understanding the nuclear charge provides a deeper insight into the nature of elements and their interactions within the world of chemistry.
Protons: The Heart of Matter
In the vast expanse of the atomic realm, lies a tiny but fundamental particle known as the proton. These positively charged denizens reside in the heart of every atom, their presence defining the element’s identity.
Protons are the cornerstone of the atomic nucleus, the central powerhouse of the atom. Within this minuscule domain, protons dance with neutrons, their combined mass determining the atom’s atomic mass. Each proton carries a significant weight of approximately one atomic mass unit (amu).
The number of protons within an atom distinguishes one element from another. This number, known as the atomic number, is a unique fingerprint for each element. For example, silver, the gleaming metal we cherish, bears an atomic number of 47. This means that each silver atom houses precisely 47 protons, granting it its distinct chemical properties.