The Ultimate Guide To Atomic Number And Its Role In Element Identity
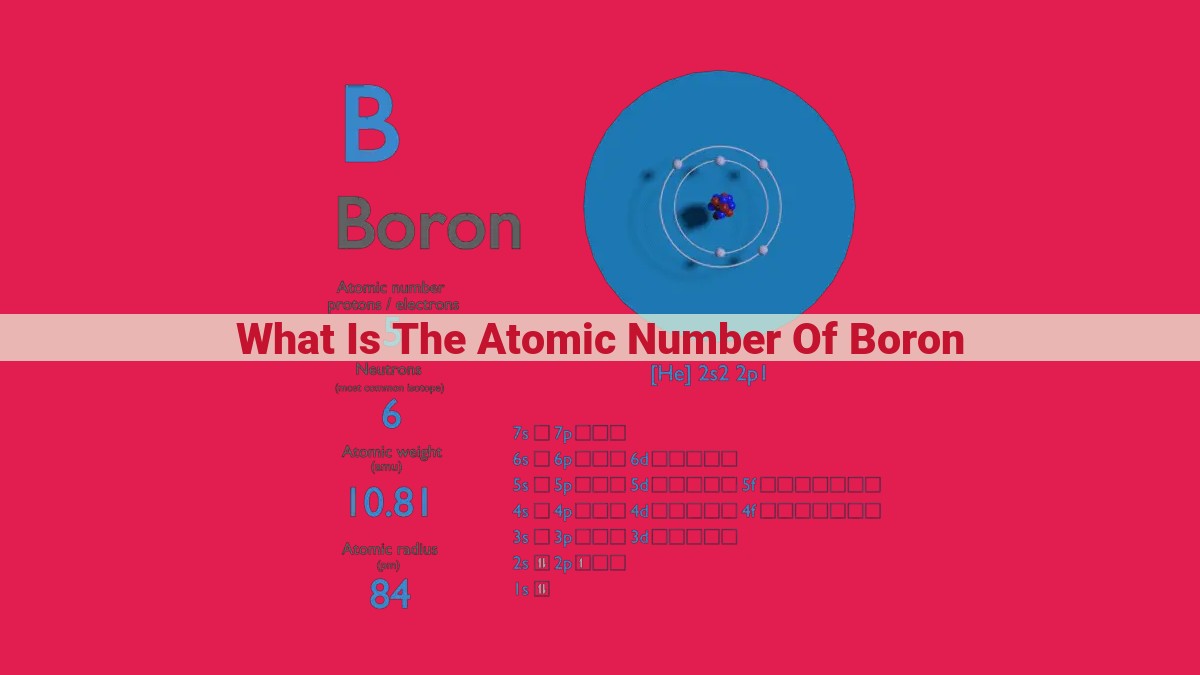
The atomic number is a fundamental property of an element that defines its identity. It is the number of protons in the nucleus of an atom. Boron is a metalloid element with atomic number 5, which means it has 5 protons in its nucleus. This information can be found in the periodic table, where boron is located in Group 13. The atomic number plays a crucial role in determining the chemical properties of an element, as it determines the number of electrons in its outermost shell.
In the vast realm of chemistry, understanding the fundamental properties of elements is crucial for unraveling the mysteries of matter. One of the most significant characteristics that sets elements apart is their atomic number.
An atomic number is like a unique fingerprint for each element. It represents the number of protons found in the nucleus of an atom. Protons, along with neutrons and electrons, are the building blocks of nature’s tiny architects. The atomic number is a defining trait that determines an element’s identity and its place in the grand scheme of things.
It’s like having a special code that tells you who’s who in the chemical world. Each element has its own unique atomic number, and this number acts as a roadmap for predicting the element’s behavior and properties. It’s a fundamental piece of information that helps us understand the intricate tapestry of chemical reactions and the substances that make up our world.
Understanding the Periodic Table: The Cornerstone of Element Organization
The periodic table, a ubiquitous tool in chemistry, serves as a visual masterpiece that organizes the known chemical elements in a way that reveals their intricate interconnections. At the heart of this organization lies atomic number, a fundamental property that distinguishes one element from another.
The Periodic Table: A Symphony of Atomic Numbers
Imagine a majestic symphony, where each note represents an element. The periodic table is akin to a musical score, arranging these “notes” in a harmonious order based on their atomic numbers. The atomic number of an element, symbolized by the letter Z, represents the number of protons found in the nucleus of its atoms. These protons, positively charged particles, define an element’s unique identity, influencing its chemical properties and behavior.
The Periodic Table: A Guide to Chemical Properties
The periodic table is not merely a static display of elements; it is a dynamic guide that predicts and explains the chemical properties of each element. By comparing the atomic numbers of different elements, we can discern patterns and relationships that shed light on their reactivity, bonding tendencies, and physical characteristics.
For instance, elements with consecutive atomic numbers tend to have similar chemical properties. This is evident in vertical columns, known as groups, which contain elements that share a common set of valence electrons. These valence electrons, located in the outermost energy level of an atom, play a crucial role in chemical bonding.
The Periodic Table: A Journey Through the Elements
As we traverse the periodic table from left to right, the atomic number increases, and the number of protons in the nucleus rises accordingly. This change in atomic number leads to a gradual shift in chemical properties. Elements on the left side of the table, known as metals, are typically malleable, ductile, and good conductors of electricity.
In contrast, elements on the right side of the table, known as nonmetals, are often brittle, poor conductors of electricity, and can form covalent bonds. This fundamental difference in properties can be attributed to the varying number of valence electrons, which influences their bonding behavior.
The periodic table stands as an invaluable tool in chemistry, providing a comprehensive framework for understanding the chemical world. By organizing elements based on their atomic numbers, it reveals the intricate relationships between their properties and behavior. As we delve deeper into the periodic table, we gain a profound appreciation for the diversity and interconnectedness of chemical elements, unlocking the secrets to countless scientific discoveries and technological advancements.
Boron: A Unique Metalloid with Atomic Number 5
In the realm of chemistry, the atomic number plays a pivotal role in shaping the identity and behavior of every element. It’s like a personal code that distinguishes one element from another, giving it its unique properties and place in the grand scheme of the periodic table.
Atomic Number: The Foundation of Element Classification
The atomic number is a fundamental characteristic of an element that defines the number of protons residing in its nucleus. This number is unique to each element and serves as the key to understanding its chemical behavior. The periodic table, a masterpiece of chemical organization, arranges elements in ascending order of their atomic numbers, creating a visual map of their similarities and differences.
Boron: A Metalloid with a Distinct Identity
Boron, an intriguing element with an atomic number of 5, stands out as a metalloid, a bridge between metals and nonmetals. This enigmatic element possesses a fascinating combination of properties, making it both unique and versatile in the chemical world.
Boron’s Position in the Periodic Table
Boron finds its home in Group 13 of the periodic table, a neighborhood of elements that share similar chemical characteristics. Its atomic number of 5 places it in the second row, indicating that it has two energy levels or shells of electrons. Boron shares the group’s affinity for forming covalent bonds, where atoms bond by sharing electrons, giving rise to a wide range of compounds with diverse applications.
The Direct Correlation: Atomic Number and Protons
The atomic number of an element is directly proportional to the number of protons in its nucleus. For boron, this means it has 5 protons in its tiny atomic heart. This number of protons determines the element’s positive charge and plays a crucial role in its chemical interactions.
Boron’s Atomic Number: 5 and its Implications
With an atomic number of 5, boron exhibits characteristics that set it apart from its neighbors in the periodic table. Its relatively low number of protons results in a less-pronounced positive charge, giving boron a unique ability to form bonds with various elements. This versatility makes it an essential component in numerous industrial processes and scientific applications.
The atomic number of an element, exemplified by boron’s atomic number of 5, serves as a fundamental pillar in the study of chemistry. It shapes the element’s identity, behavior, and position within the periodic table. Understanding atomic numbers empowers us to predict and harness the properties of elements, unlocking the potential for scientific advancements and technological innovations.
Boron’s Position in the Periodic Table
As we journey through the fascinating realm of chemistry, the periodic table emerges as our guiding compass. It organizes elements based on their atomic numbers, revealing the hidden patterns and relationships that govern their behavior. In this pilgrimage, we shall uncover the unique position of boron, an enigmatic element that resides in the heart of the periodic table.
Group 13, a family of elements known for their metalloid nature, welcomes boron with open arms. These elements share a common trait: they possess three valence electrons, the outermost electrons that determine their chemical reactivity. Boron, with its atomic number of 5, proudly boasts three valence electrons, granting it a unique place among its siblings.
Within Group 13, boron exhibits a captivating camaraderie with its fellow elements. It shares a chemical kinship with aluminum, gallium, indium, and thallium, all of whom don the mantle of metalloids. This shared lineage translates into similar chemical properties, making them a cohesive family within the periodic table’s embrace.
Boron’s position in Group 13 not only defines its chemical nature but also shapes its destiny. The three valence electrons that characterize its atomic structure endow boron with a remarkable ability to form covalent bonds, establishing bonds with other atoms by sharing electron pairs. This covalent bonding tendency plays a pivotal role in shaping boron’s chemical behavior and versatility.
As we delve deeper into the periodic table, we uncover a compelling narrative that weaves together atomic numbers, group placements, and chemical properties. Boron’s position in Group 13 serves as a testament to the intricate dance between these elements, showcasing the power of the periodic table to unravel the secrets of the chemical world.
The Intimate Connection Between Atomic Number and Protons: Unlocking the Secrets of Matter
At the heart of every atom lies a fundamental concept that governs its identity and behavior: atomic number. This numerical value holds the key to understanding the very essence of elements and their fascinating interactions.
Imagine an atom as a miniature solar system, with the nucleus as its central star and electrons orbiting like planets. Within this tiny cosmic realm, the atomic number plays a pivotal role in defining the atom’s character. It represents the number of protons residing within the nucleus, the positively charged particles that determine an element’s unique position on the periodic table.
Just as each planet in our solar system has a specific number of moons, each element in the universe possesses a distinct atomic number. This number is not arbitrary but rather a fundamental property that governs the element’s chemical behavior, reactivity, and place among its fellow elements.
The periodic table, that iconic grid of elements, is arranged according to atomic numbers. Elements with the same atomic number share similar chemical properties, forming vertical columns known as groups. By tracing down a group, we observe a gradual increase in atomic number, which corresponds to an increase in the number of protons in the nucleus.
For instance, let’s take the element boron, a metalloid with an atomic number of 5. This means that every boron atom contains exactly 5 protons in its nucleus. This defining characteristic places boron in Group 13 of the periodic table, where it shares chemical affinities with other elements that also have 5 protons per atom.
Boron: The Element with Atomic Number 5
In the captivating world of chemistry, atomic number takes center stage, distinguishing one element from another. This crucial number, which represents the number of protons in an atom’s nucleus, holds the key to understanding the properties and behavior of elements.
Boron, a metalloid element, occupies a special place in the periodic table. Its atomic number, a mere 5, reveals fascinating insights into its nature. This number signifies that every boron atom contains five protons in its nucleus. By understanding this fundamental characteristic, we can unravel the secrets that make boron unique.
The atomic number of an element has profound implications for its chemical properties. In the case of boron, its position in Group 13 of the periodic table alongside aluminum and gallium indicates a shared propensity for forming compounds with three valence electrons. This shared feature underlies the remarkable chemical versatility of these elements.
Moreover, boron’s atomic number of 5 enables it to participate in diverse chemical reactions. For instance, its ability to form covalent bonds with itself and other elements, such as hydrogen and oxygen, contributes to its wide range of applications. From the production of boron fibers for aerospace and sporting equipment to the manufacture of borosilicate glass for laboratory glassware and cookware, boron’s unique properties find countless uses in various industries.
In conclusion, the atomic number of an element serves as a compass, guiding us through the vast landscape of chemistry. By understanding the atomic number of boron as 5, we gain a deeper appreciation for its exceptional properties and its crucial role in numerous technological advancements. This knowledge empowers us to harness the potential of boron and other elements to shape the future of science and engineering.