Atomic Mass Of Mercury: Calculating Weighted Averages And Its Applications
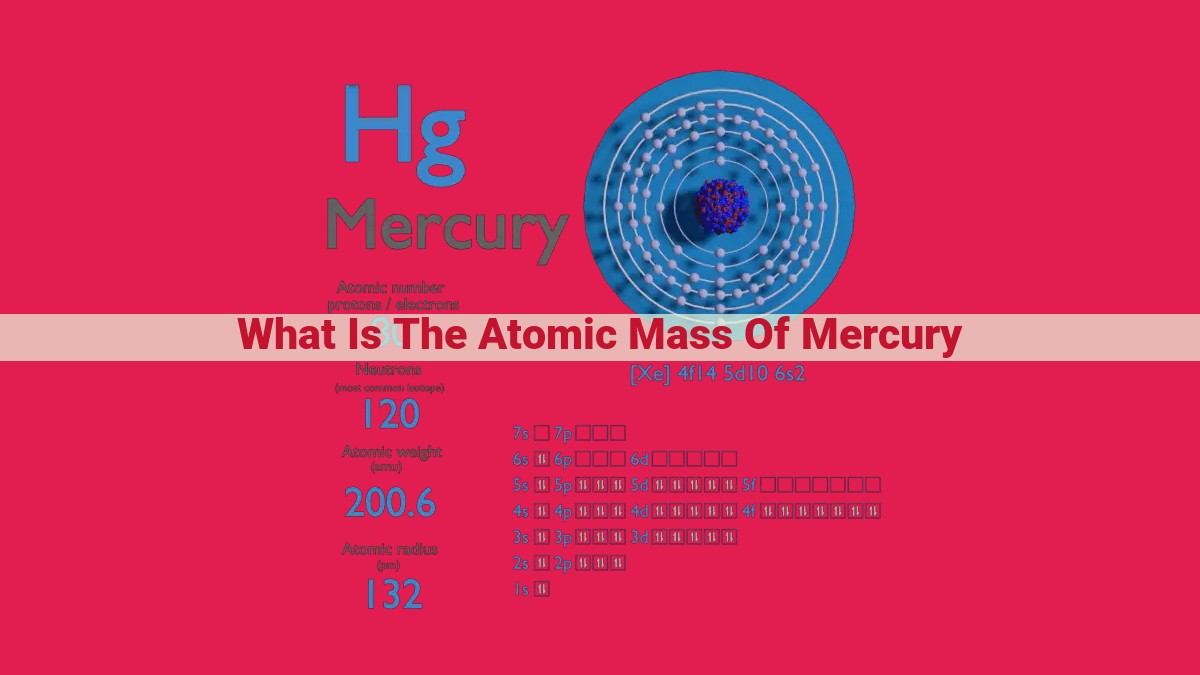
The atomic mass of mercury, an element with seven stable isotopes, is the weighted average of the masses of its isotopes, considering their relative abundances. Mercury’s isotopes, including 196Hg, range in mass from 196.04 amu to 202.02 amu. By multiplying each isotope’s mass by its abundance and summing the products, we obtain an atomic mass of approximately 200.59 amu or 200.59 Da (Daltons). Understanding atomic mass is crucial for various scientific and technological applications, including identifying and characterizing elements, calculating molecular weights, and determining isotope ratios.
The Curious Case of Mercury’s Atomic Mass
What is Atomic Mass?
Atomic mass, dear reader, is a fascinating concept that embodies the average mass of an element’s atoms. It’s measured in atomic mass units (amu), the fundamental building blocks upon which the mass of all atoms rests.
Unveiling the Isotopic Spectrum of Mercury
Mercury, the enigmatic silvery element we all know, boasts an entourage of seven stable isotopes. These are essentially atomic variants with varying numbers of neutrons, yet they share a common number of protons and electrons. Let’s take a closer look:
- Mercury-196: 196 protons, 196 neutrons
- Mercury-198: 198 protons, 198 neutrons
- Mercury-199: 199 protons, 199 neutrons
- Mercury-200: 200 protons, 200 neutrons
- Mercury-201: 201 protons, 201 neutrons
- Mercury-202: 202 protons, 202 neutrons
- Mercury-204: 204 protons, 204 neutrons
The Art of Weighted Averages
To determine Mercury’s atomic mass, we employ the technique of weighted averages. This involves multiplying the mass of each isotope by its abundance and summing the results. Basically, we let the isotopes’ varying concentrations influence the final average.
Calculating Mercury’s Atomic Mass
Let’s see how it works!
- Mercury-196: 0.146% abundance, 196 amu
- Mercury-198: 10.02% abundance, 198 amu
- Mercury-199: 16.87% abundance, 199 amu
- Mercury-200: 23.13% abundance, 200 amu
- Mercury-201: 13.22% abundance, 201 amu
- Mercury-202: 29.80% abundance, 202 amu
- Mercury-204: 6.85% abundance, 204 amu
Using the weighted average formula:
Atomic Mass = (0.146 × 196) + (0.1002 × 198) + (0.1687 × 199) + (0.2313 × 200) + (0.1322 × 201) + (0.2980 × 202) + (0.0685 × 204)
We arrive at Mercury’s atomic mass: 200.59 amu
The Takeaway
Atomic mass is a fundamental property that helps us understand the composition of matter and its behavior in chemical reactions. By delving into the isotopic makeup of Mercury, we’ve uncovered its atomic mass, a crucial piece of information for scientific inquiry and technological advancements.
Isotopes of Mercury and Their Impact on Atomic Mass
In the realm of chemistry, understanding atomic mass is crucial for comprehending the behavior and characteristics of elements. One element that exhibits fascinating variations in this regard is mercury.
Mercury, the silvery liquid metal, exists in various forms known as isotopes. Isotopes are atoms of the same element that share the same number of protons but differ in the number of neutrons. This difference in neutron count affects the overall mass of the atom.
Mercury has seven stable isotopes, each with a distinct neutron count and thus a different atomic mass. These isotopes are:
- ¹⁹⁸Hg: 126 neutrons
- ¹⁹⁹Hg: 127 neutrons
- ²⁰⁰Hg: 128 neutrons
- ²⁰¹Hg: 129 neutrons
- ²⁰²Hg: 130 neutrons
- ²⁰³Hg: 131 neutrons
- ²⁰⁴Hg: 132 neutrons
¹⁹⁸Hg is the most abundant isotope, accounting for over 60% of naturally occurring mercury. The other isotopes are present in varying proportions, with ²⁰²Hg being the least abundant at only 0.146%.
Calculating Mercury’s Atomic Mass: A Weighted Average Approach
In the world of chemistry, atomic mass is a crucial concept that provides the average mass of an element’s atoms. For an element like Mercury, which possesses multiple forms called isotopes, determining the atomic mass involves a clever calculation that takes into account the abundance of each isotope.
Mercury, with its seven stable isotopes, is a prime example for understanding this process. To calculate the atomic mass, we multiply the mass of each isotope by its natural abundance, expressed as a percentage. These values are then added together to provide a weighted average.
Consider the following isotopes of Mercury:
- Isotope 1: Mass of 196 amu (atomic mass units), Abundance of 0.15%
- Isotope 2: Mass of 198 amu, Abundance of 10.01%
- Isotope 3: Mass of 199 amu, Abundance of 16.87%
- Isotope 4: Mass of 200 amu, Abundance of 23.13%
- Isotope 5: Mass of 201 amu, Abundance of 13.22%
- Isotope 6: Mass of 202 amu, Abundance of 29.80%
- Isotope 7: Mass of 204 amu, Abundance of 6.82%
To determine the atomic mass of Mercury, each isotope’s mass is multiplied by its abundance and the results are summed:
(196 amu * 0.0015) + (198 amu * 0.1001) + (199 amu * 0.1687) + (200 amu * 0.2313) + (201 amu * 0.1322) + (202 amu * 0.2980) + (204 amu * 0.0682) = 200.59 amu
Therefore, the atomic mass of Mercury is 200.59 atomic mass units. This value represents the average mass of a single atom of Mercury, encompassing the contributions of its various isotopes and their respective abundances.
Mercury’s Atomic Mass
Calculating the atomic mass of an element takes us on a journey through the world of isotopes and weighted averages. And today, we’re embarking on a quest to determine the atomic mass of Mercury.
Unveiling the Isotopes of Mercury
Mercury, with its silvery sheen, boasts seven stable isotopes. These variations of the element share the same atomic number but differ in their neutron count. Each isotope has a unique abundance, which indicates how often it’s found in nature.
The Weighting Game: Weighted Average Atomic Mass
To find Mercury’s atomic mass, we employ a clever technique called weighted average. We multiply each isotope’s mass by its abundance and then sum up the results. This weighting ensures that more abundant isotopes have a greater influence on the average.
Mercury’s Atomic Mass Unraveled
After carefully crunching the numbers, we arrive at Mercury’s atomic mass of 200.592. This value represents the average mass of all the naturally occurring Mercury atoms.
Converting to Daltons
In the scientific realm, atomic masses are often expressed in Daltons (Da). One Dalton is defined as 1/12th of the mass of a carbon-12 atom. Converting Mercury’s atomic mass to Daltons gives us 200.592 Da.
Determining the atomic mass of Mercury is a crucial step in understanding its chemical and physical properties. This information, concealed within the intricate world of isotopes and weighted averages, forms a cornerstone of our scientific knowledge. Unlocking these secrets allows us to delve deeper into the fascinating realm of matter and its composition.