Unlocking The Atomic Mass Of Copper: Unraveling Its Role In Properties And Behavior
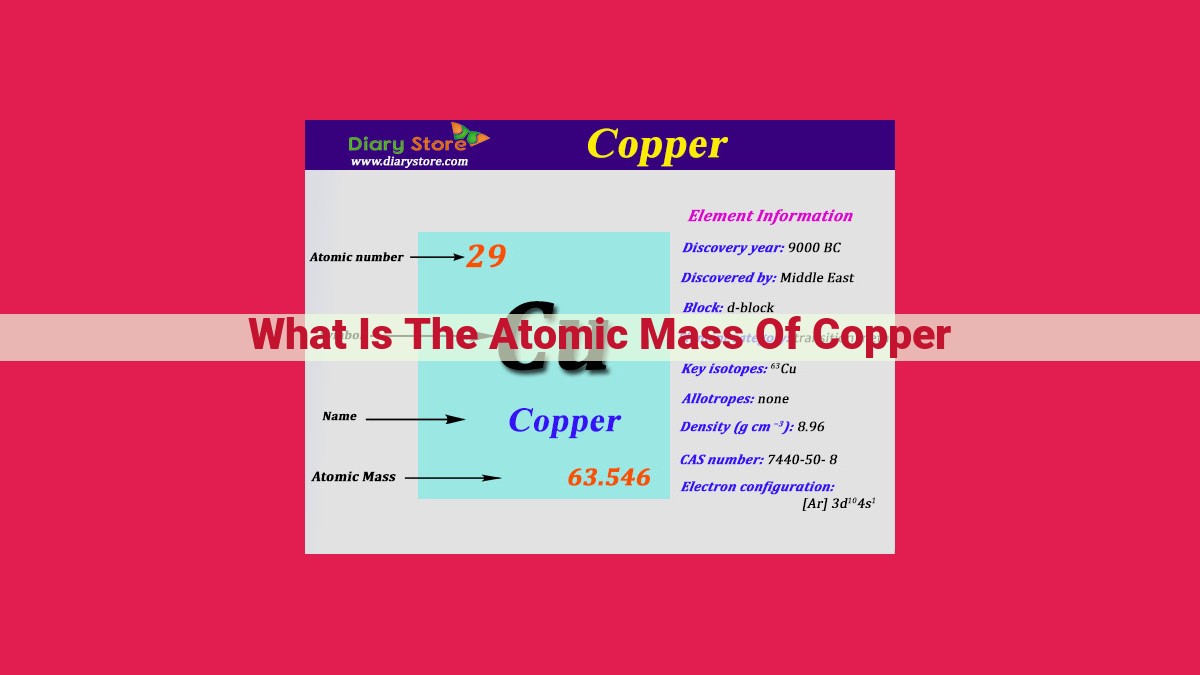
The atomic mass of copper is 63.55 atomic mass units (amu). This weighted average represents the contributions of copper’s two stable isotopes: 63Cu (69.17% abundance, 62.93 amu) and 65Cu (30.83% abundance, 64.93 amu). Copper’s atomic mass helps determine its physical and chemical properties, such as density and thermal conductivity. Understanding the atomic mass of elements is essential for comprehending their behavior and predicting their reactivity.
Atomic Mass: A Fundamental Concept in Chemistry
In the vast world of chemistry, understanding the atomic mass of an element is crucial for grasping its properties and behavior. Atomic mass is the weighted average of the masses of all the isotopes of an element. Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons.
The atomic mass of an element provides insights into its physical and chemical characteristics. It influences factors such as density, thermal conductivity, and reactivity. Understanding these properties enables scientists and researchers to predict and control the behavior of elements in various chemical reactions and applications.
Concept of Atomic Mass: A Deeper Dive
Atomic mass, the cornerstone of chemistry, plays a pivotal role in unraveling the mysteries of elements and their properties. It is the weighted average of the masses of an element’s isotopes, taking into account their relative abundances.
Isotopes, like siblings in an atomic family, share the same number of protons and electrons but differ in their number of neutrons. This variation in neutron count influences their mass. For instance, take copper, our case study element. It has two stable isotopes: 63Cu and 65Cu.
The weighted average concept is crucial in determining atomic mass. Imagine a scale where each isotope’s mass is represented by a weight proportionate to its abundance. The average position of the scale when balanced gives us the atomic mass. For copper, the calculation looks like this:
Atomic mass = (63Cu mass * 63Cu abundance) + (65Cu mass * 65Cu abundance)
This calculation yields 63.55 atomic mass units (amu), the atomic mass of copper.
Atomic mass is not just a number; it carries profound implications for element properties. It influences their density, as heavier atoms pack closer together. For example, copper’s high atomic mass contributes to its exceptional density, making it useful in electrical wiring and heat sinks.
Furthermore, atomic mass affects thermal conductivity, the ability to transfer heat. Heavier atoms vibrate less, resulting in lower thermal conductivity. Copper’s relatively low thermal conductivity makes it an effective material for heat exchangers and cookware.
Understanding atomic mass is like unlocking a secret language that reveals the hidden characteristics of elements. By deciphering this language, scientists gain insights into the inner workings of matter and its endless applications in our technological world.
Case Study: Copper
- Define copper as an element with a specific atomic number and symbol.
- Introduce copper’s two stable isotopes, 63Cu and 65Cu.
Copper: Unveiling the Secrets of Atomic Mass
Copper, with the atomic number 29 and symbol Cu, is a fascinating element with a unique atomic mass that plays a crucial role in shaping its properties. Copper has two naturally occurring stable isotopes: 63Cu and 65Cu. Isotopes are forms of the same element with identical atomic numbers but differing neutron counts, resulting in different masses.
In the case of copper, 63Cu is the more abundant isotope, accounting for 69.17% of naturally occurring copper atoms. It has an atomic mass of approximately 62.93 atomic mass units (amu). The less abundant isotope, 65Cu, comprises 30.83% of copper atoms and has an atomic mass of approximately 64.93 amu.
Understanding the concept of atomic mass is essential in comprehending the behavior of elements like copper. Atomic mass represents the weighted average mass of an element’s naturally occurring isotopes. To calculate the atomic mass of an element, we multiply the mass of each isotope by its abundance and then add the products together.
In the case of copper, the atomic mass can be calculated as follows:
Atomic mass (Cu) = (62.93 amu x 0.6917) + (64.93 amu x 0.3083)
This calculation gives us an atomic mass of approximately 63.55 amu for copper. This number provides valuable insights into the average mass of a copper atom and the relative contributions of its isotopes.
Calculating Copper’s Atomic Mass: A Closer Look
In the world of chemistry, understanding the atomic mass of an element is crucial for comprehending its properties and behavior. And when it comes to copper, a versatile metal widely used in countless applications, its atomic mass holds particular significance.
Copper, denoted by the symbol Cu, is an element with an atomic number of 29. It exists in two stable isotopic forms: 63Cu and 65Cu. To determine the atomic mass of copper, we need to take into account both its isotopes and their respective abundances.
The atomic mass of an element is the weighted average of the masses of its isotopes. This means that we multiply the mass of each isotope by its abundance and then add the results together. For copper, the abundance of 63Cu is 69.17%, while the abundance of 65Cu is 30.83%.
Using the weighted average formula:
Atomic mass = (mass of isotope 1 x abundance of isotope 1) + (mass of isotope 2 x abundance of isotope 2)
We can calculate copper’s atomic mass:
Atomic mass = (62.93 amu x 0.6917) + (64.93 amu x 0.3083)
Atomic mass = 43.56 amu + 19.99 amu
Atomic mass = 63.55 amu
Therefore, the atomic mass of copper is determined to be 63.55 amu. This value provides us with valuable insights into copper’s properties, such as its density and thermal conductivity, which are influenced by its mass and the arrangement of its atoms.
Implications of Atomic Mass in Copper’s Properties
Copper, an element with atomic number 29, exhibits unique physical and chemical characteristics influenced by its atomic mass, a crucial factor in understanding its behavior. The atomic mass of copper, approximately 63.55 atomic mass units (amu), plays a pivotal role in shaping its properties.
Copper’s high density is directly linked to its atomic mass. The mass of its atoms contributes to the overall density of the substance. A denser material is harder to compress, making copper resistant to deformation. This property makes it a suitable choice for wiring, where structural integrity is paramount.
The thermal conductivity of copper is another feature influenced by its atomic mass. The heavier atoms in copper impede the flow of heat, resulting in excellent thermal conductivity. This property makes copper an efficient conductor of heat, enabling its use in heat sinks and electrical components where efficient heat dissipation is essential.
In summary, the atomic mass of copper is a crucial factor in determining its properties. Its high density contributes to its structural stability, while its excellent thermal conductivity makes it a valuable material for applications requiring efficient heat dissipation. Understanding the implications of atomic mass helps us appreciate the intricate interplay between atomic structure and material properties, providing valuable insights into the behavior of elements like copper.