Aspirin: Understanding Its Molecular Structure For Seo
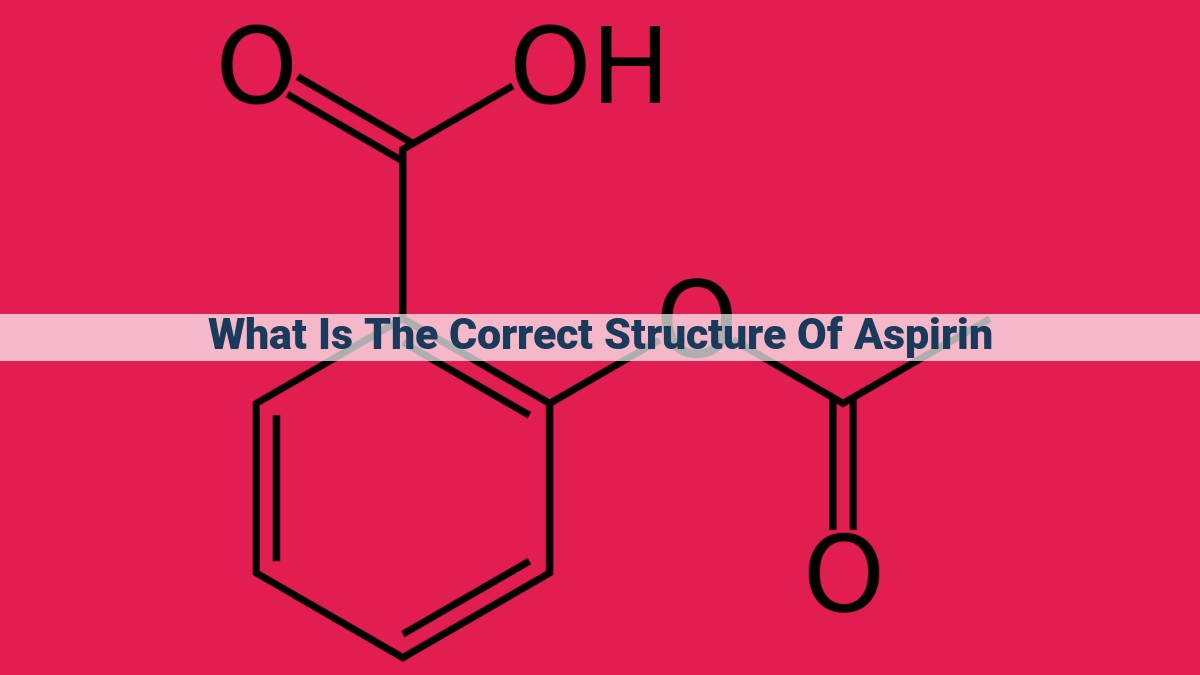
Aspirin, a renowned pain reliever, comprises a salicylic acid core with an acetyl group attached. The salicylic acid exhibits a carboxylic acid group, a benzene ring, and a hydroxyl group, while the acetyl group consists of a methyl group and a carbonyl group. The carboxylic acid group and the acetyl group form an ester linkage, creating aspirin’s distinct molecular structure.
Salicylic Acid: The Cornerstone of Aspirin
In the realm of medicine, few ingredients hold as much historical significance as salicylic acid. This unassuming compound is the keystone in the creation of one of the world’s most widely used pain relievers: aspirin.
Delving into the Molecular Makeup of Salicylic Acid
Salicylic acid is an organic compound with a distinctive chemical structure. It boasts a carboxylic acid group, a hydroxyl group, and a benzene ring. The carboxylic acid group is the heart of the molecule, providing it with acidic properties. The hydroxyl group adds a level of reactivity, while the benzene ring serves as a stable foundation.
The Role of Acetic Anhydride: A Chemical Catalyst
When salicylic acid embarks on its journey to becoming aspirin, it encounters acetic anhydride, an essential catalyst in this chemical transformation. Acetic anhydride plays a crucial role in the acetylation reaction, aiding in the addition of an acetyl group to salicylic acid. This addition marks the birth of aspirin.
Introducing Aspirin: The Culmination of a Chemical Dance
Aspirin is the end product of this meticulous chemical process. Its molecular structure showcases the acetylation of the carboxylic acid group in salicylic acid. This subtle change bestows upon aspirin its characteristic properties, making it an effective pain reliever and fever reducer.
Unveiling the Significance of the Carboxylic Acid Group
The carboxylic acid group plays a multifaceted role in both salicylic acid and aspirin. In salicylic acid, it contributes to the compound’s acidic nature. In aspirin, it forms an ester linkage with the acetyl group, a crucial bond that underpins aspirin’s therapeutic effects.
The Acetyl Group: A Vital Addition
The acetyl group, added during the acetylation reaction, is a small but potent molecular entity. It not only alters the chemical properties of salicylic acid but also enhances its therapeutic value, making it the pain-relieving powerhouse we know as aspirin.
The Ester Linkage: A Chemical Bridge
The ester linkage formed between the carboxylic acid group and the acetyl group is a testament to the power of chemical bonding. This linkage creates a stable molecular structure, ensuring that aspirin retains its effectiveness over time.
The Benzene Ring: A Stable Foundation
The benzene ring in salicylic acid and aspirin provides a stable framework for these molecules. This ring structure, composed of six carbon atoms arranged in a hexagonal shape, contributes to the overall stability and rigidity of both compounds.
The Hydroxyl Group: A Functional Detail
The hydroxyl group in salicylic acid plays a subtle but important role. It influences the solubility and reactivity of the molecule, adding an extra layer of complexity to its chemical makeup.
Understanding the chemical structure of salicylic acid and its role in the production of aspirin not only enhances our appreciation for this ubiquitous pain reliever but also underscores the significance of chemistry in shaping the medical landscape. It is through the precise manipulation of molecular structures that we unlock the healing power of compounds like salicylic acid and aspirin.
Acetic Anhydride: The Magician Behind Aspirin
In the realm of medicine, aspirin reigns supreme as one of the most widely used pain relievers. But have you ever wondered about the intricate chemical dance that brings this wonder drug to life? Acetic anhydride, a colorless liquid, plays a crucial role in this fascinating process.
Acetylation: The Magic Touch
The transformation of salicylic acid into aspirin involves a reaction known as acetylation, and acetic anhydride is the catalyst that makes it all happen. This colorless liquid acts as an acetylation agent, which means it donates an acetyl group to salicylic acid, giving rise to aspirin.
The acetyl group is like a small chemical cloak that wraps around the carboxylic acid group of salicylic acid, altering its properties and giving birth to aspirin. This addition not only enhances aspirin’s pain-relieving capabilities but also improves its stability and solubility.
Behind the Scenes: The Reaction Unfolds
The acetylation reaction can be likened to a chemical ballet, where acetic anhydride gracefully interacts with salicylic acid. The carboxylic acid group of salicylic acid, like a waiting partner, eagerly awaits the acetyl group brought by acetic anhydride. As they gracefully combine, an ester linkage is formed, the chemical bond that unites them.
This ester linkage is the lynchpin that holds aspirin together, giving it its unique molecular structure and properties. It’s not just a chemical bond; it’s the very essence of aspirin’s existence.
The Power of Partnership
The success of aspirin lies not only in its individual components but also in the harmonious partnership between salicylic acid and acetic anhydride. Acetic anhydride, the acetylation agent, breathes life into salicylic acid, transforming it into the potent pain reliever we know and trust.
Without this crucial acetylation reaction, the world would be deprived of one of its most valuable medical advancements. So, let us raise a toast to acetic anhydride, the behind-the-scenes hero that makes aspirin’s existence possible.
Aspirin: The Final Product
- Discuss the molecular structure of aspirin, highlighting the addition of the acetyl group to the carboxylic acid group.
Aspirin: The Final Product
In the realm of pharmaceuticals, aspirin stands as a testament to the transformative power of chemistry. Born from the humble salicylic acid, this ubiquitous pain reliever owes its existence to a remarkable chemical reaction that alters its molecular structure.
Acetylation: The Key to Transformation
At the heart of aspirin’s creation lies acetylation, a process that deftly adds an acetyl group to the carboxylic acid group of salicylic acid. Like a master craftsman, acetic anhydride, an acetylation agent, orchestrates this crucial transformation.
The Molecular Blueprint of Aspirin
As the acetyl group gracefully bonds with the carboxylic acid group, an ester linkage emerges, creating the aspirin molecule. This newly formed ester linkage becomes a pivotal point in aspirin’s structure, bridging the gap between the two functional groups.
A Stable Scaffold: The Benzene Ring
Embedded within the aspirin molecule is the benzene ring, a resilient structure that lends stability to the compound. Its six carbon atoms form a hexagonal framework, contributing to aspirin’s chemical longevity.
The Hydroxyl Group: A Subtle Addition
Salicylic acid also bears a hydroxyl group, a feature that enhances its water solubility. Though not directly involved in aspirin’s pain-relieving properties, this hydroxyl group adds a touch of chemical versatility.
Aspirin: A Medical Marvel
Through the ingenious fusion of chemistry and medicine, salicylic acid transforms into aspirin, a potent pain reliever that has alleviated suffering for generations. Its molecular structure, a testament to the power of chemical manipulation, speaks volumes about the intricate dance of atoms and the profound impact it can have on human health.
The Role of the Carboxylic Acid Group
- Explain the concept of the carboxylic acid group, its presence in both salicylic acid and aspirin, and its involvement in the ester linkage.
The Carboxylic Acid Group’s Role in Aspirin Formation
To fully understand the extraordinary power of aspirin, we must delve into the hidden world of its molecular makeup, where the carboxylic acid group plays a crucial role in its creation and effectiveness.
What is a Carboxylic Acid Group?
Imagine a molecule with a carbon atom surrounded by two oxygen atoms and a hydrogen atom. This is the fundamental structure of a carboxylic acid group, a highly reactive functional group that grants molecules their acidic properties.
Carboxylic Acid Group in Salicylic Acid
Salicylic acid, the precursor to aspirin, boasts a carboxylic acid group attached to a benzene ring. This group is responsible for salicylic acid’s acidity and its ability to react with other molecules.
Carboxylic Acid Group in Aspirin
When salicylic acid undergoes a chemical reaction with acetic anhydride, the carboxylic acid group plays a pivotal role. It reacts with the acetyl group from the acetic anhydride to form an ester linkage, a chemical bond that unites the two molecules.
The Ester Linkage: A Bridge to Relief
This newly formed ester linkage is the cornerstone of aspirin’s existence. The acetyl group, derived from acetic anhydride, effectively neutralizes the acidic nature of the carboxylic acid group, transforming salicylic acid into the more tolerable and effective compound we know as aspirin.
The Acetyl Group: A Key Addition in Aspirin Synthesis
In the realm of pain relievers, aspirin stands tall as a trusted remedy. Its remarkable effectiveness owes much to a crucial ingredient: the acetyl group. This tiny molecular fragment plays a transformative role in the journey from salicylic acid to aspirin.
The acetyl group, with its simple yet potent structure, consists of a carbon atom flanked by two oxygen atoms, forming a carboxylic acid group. When it encounters salicylic acid, a chemical dance ensues, culminating in the acetylation reaction. This reaction involves the formation of an ester linkage between the carboxylic acid group of salicylic acid and the acetyl group.
The acetyl group’s addition to salicylic acid is not merely a cosmetic change. It imparts a profound shift in the molecule’s character. By masking the acidic nature of salicylic acid, the acetyl group reduces its irritating effects on the stomach lining, making aspirin more tolerable.
Furthermore, the ester linkage formed between the acetyl group and the salicylic acid stabilizes the molecule. This increased stability is crucial for aspirin’s ability to withstand the acidic environment of the stomach and reach its target site in the body where it exerts its pain-relieving effects.
In essence, the acetyl group acts as a chemical bridge, connecting the carboxylic acid group of salicylic acid to the hydroxyl group of acetic anhydride, thereby completing the synthesis of aspirin. This seemingly small molecular modification transforms salicylic acid from a harsh acid to a gentler and more effective pain reliever.
The Ester Linkage: A Chemical Bridge in Aspirin
In the realm of chemistry, the ester linkage plays a pivotal role in the synthesis of aspirin, a marvel of modern medicine. Aspirin, renowned for its analgesic and antipyretic properties, owes its existence to a crucial chemical transformation known as acetylation. This process involves the union of two molecules: salicylic acid, the primary ingredient in aspirin, and acetic anhydride, an acetylating agent.
The ester linkage, a robust chemical bond, is forged between the carboxylic acid group of salicylic acid and the acetyl group of acetic anhydride. The carboxylic acid group, with its characteristic -COOH structure, resembles a tiny magnet, attracting electrons from neighboring atoms. The acetyl group, on the other hand, is a potent electron donor, eager to share its electrons.
When these two chemical entities encounter each other, a captivating dance ensues. The electron-hungry carboxylic acid group reaches out to the generous acetyl group, forming a covalent bond between the carbon atom of the acetyl group and the oxygen atom of the carboxylic acid group. This intimate embrace creates an ester linkage, a bridge that connects the two molecules.
The formation of the ester linkage marks the birth of aspirin. It transforms salicylic acid from a relatively weak acid into a more potent one, enhancing its medicinal properties. This simple yet profound chemical modification empowers aspirin with its ability to combat pain, fever, and inflammation.
The ester linkage, a testament to the power of chemical bonding, lies at the heart of aspirin’s remarkable effectiveness. It is a molecular masterpiece that bridges the gap between chemistry and medicine, bringing relief to countless individuals worldwide.
The Benzene Ring: A Sturdy Foundation
In the molecular tapestry of aspirin, the benzene ring stands as an unyielding bastion, lending an air of stability to this wonder drug. Found in both salicylic acid and aspirin, this hexagonal structure, composed of six carbon atoms, defiantly resists chemical change.
The benzene ring’s resilience stems from its unique resonance, where electrons dance in a perpetual waltz, delocalizing across the ring. This electron-sharing creates an extraordinarily stable molecular framework, enabling the ring to withstand the rigors of chemical reactions.
In the realm of aspirin, the benzene ring forms the backbone of the molecule, anchoring the acetyl group and carboxylic acid group. Its steadfast presence ensures aspirin’s durability and effectiveness, making it a time-honored remedy for pain and inflammation.
The Hydroxyl Group: A Functional Detail
Nestled within the molecular structure of salicylic acid lies the hydroxyl group, an entity that may seem inconspicuous but plays a pivotal role in shaping the chemical properties of this aspirin precursor. Situated at the periphery of the salicylic acid molecule, the hydroxyl group consists of an oxygen atom covalently bonded to a hydrogen atom. This functional group imparts unique characteristics to salicylic acid, influencing its solubility, acidity, and reactivity.
The hydroxyl group’s location on the benzene ring of salicylic acid is strategic. It is adjacent to the carboxylic acid group, another key functional group, forming an intramolecular hydrogen bond. This hydrogen bond strengthens the molecular structure, making salicylic acid more stable and less reactive than other similar compounds.
Moreover, the hydroxyl group is responsible for salicylic acid’s weakly acidic nature. The hydrogen atom in the hydroxyl group is slightly acidic and can be donated to a base, forming a salt. This acidity allows salicylic acid to dissolve in water and makes it suitable for use in various applications, including pain relievers and skincare products.
The hydroxyl group’s influence extends beyond salicylic acid’s physical properties. It also plays a crucial role in chemical reactions, particularly in the formation of aspirin. In the acetylation reaction, acetic anhydride reacts with the hydroxyl group of salicylic acid, leading to the formation of an ester linkage. This linkage connects the salicylic acid molecule to an acetyl group, ultimately transforming salicylic acid into the widely used analgesic, aspirin.
In summary, the hydroxyl group in salicylic acid is a multifaceted functional group that contributes to its molecular structure, acidity, solubility, and reactivity. Its presence is essential for the formation of aspirin, highlighting its significance in both the chemical and pharmaceutical realms.