Argon’s Proton Count: Exploring The Significance Of Atomic Number 18
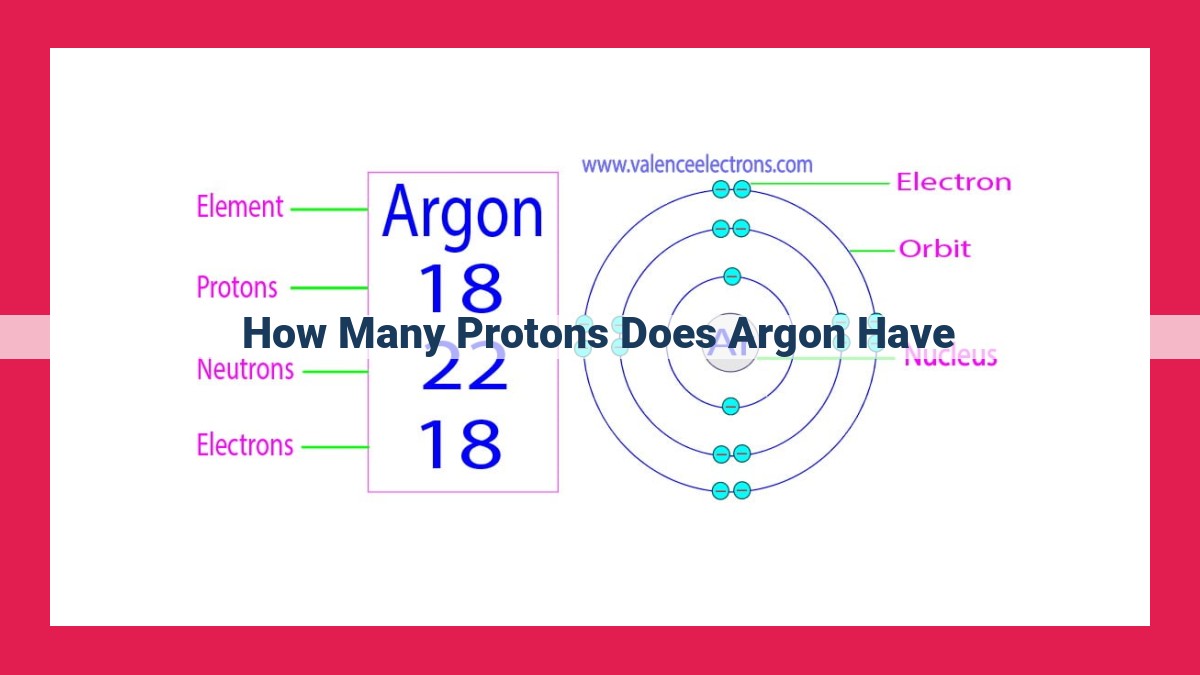
Argon, an element in the periodic table, possesses a unique atomic number of 18. This indicates that each argon atom contains 18 protons within its nucleus. The number of protons in an atom plays a significant role in defining its identity and chemical behavior. Argon’s 18 protons contribute to its stable, non-reactive nature as a noble gas. Understanding proton count is essential in nuclear physics and atomic structure, where it helps classify elements and determine their properties.
Delving into the Heart of Atoms: Argon’s Secrets Unveiled
Every atom, the fundamental building block of all matter, holds a unique identity defined by its atomic number. This number represents the count of positively charged particles known as protons nestled within the atom’s core, the nucleus. For argon, an element that graces our planet’s atmosphere, its atomic number of 18 reveals a critical aspect of its nature.
The number of protons plays a pivotal role in shaping an element’s identity and behavior. Protons carry a positive charge, which influences the atom’s chemical properties. In argon’s case, its 18 protons give it a neutral overall charge, making it a noble gas—a group of elements renowned for their low reactivity.
Understanding the number of protons in an element is essential for comprehending its place within the periodic table, the iconic chart that organizes elements based on their properties. Argon’s position in the noble gas column highlights its unique characteristics, including its lack of reactivity, which makes it invaluable in various industrial applications.
Understanding Argon’s Unique Properties: A Journey through the Periodic Table
In the vast realm of elements, argon stands out as a captivating enigma. Its noble gas status and unparalleled low reactivity have intrigued scientists for centuries. But what lies at the heart of argon’s extraordinary nature? To unravel this mystery, we embark on an enthralling voyage through the Periodic Table, the enigmatic guide that organizes the elements of the universe.
Noble Gases: The Enigma of Inertness
Argon belongs to the illustrious group of noble gases, characterized by their indifference to chemical unions. Unlike their more reactive counterparts, these gaseous wonders prefer a life of solitude, rarely engaging in chemical bonds. This aloofness stems from their full valence electron shells, rendering them content and unreceptive to sharing or exchanging electrons.
Position in the Periodic Table: A Clue to Properties
The Periodic Table is not merely a grid of elements; it is a roadmap to their properties. Argon’s placement in Group 18 reveals a crucial clue to its inertness. This group houses other noble gases, each with its distinctive atomic number but sharing the common trait of a complete valence electron shell.
Classification and Organization: The Periodic Table’s Wisdom
The Periodic Table is not just a catalog; it is a testament to the order that governs the universe. By arranging elements based on their atomic numbers and recurring chemical properties, the Periodic Table unveils patterns and relationships that guide our understanding of chemistry. Argon’s position within this magnificent scheme underscores the profound influence of electron configuration on an element’s behavior.
Through our exploration of Argon’s properties and its place in the Periodic Table, we have gained invaluable insights into the nature of this fascinating element. Its noble gas status and low reactivity are intimately linked to its unique electron configuration, a revelation that underscores the profound power of the Periodic Table as a tool for understanding the chemical world.
Nuclear Physics and Atomic Structure: Unraveling the Secrets of Protons
The world of physics is filled with intricate connections, and one such link exists between nuclear physics and our understanding of proton behavior. Protons, tiny subatomic particles residing in the heart of atoms, play a pivotal role in shaping the properties and characteristics of elements.
To delve into this intriguing realm, let’s explore the fundamental atomic structure. The atom, the basic building block of matter, is a fascinating entity composed of three primary components:
- Protons: Positively charged particles found in the nucleus, the central core of the atom.
- Electrons: Negatively charged particles that orbit the nucleus in defined energy levels.
- Neutrons: Uncharged particles, also found in the nucleus, that contribute to the atom’s mass.
The arrangement and number of these components greatly influence the properties of an element. The number of protons in an atom, known as its atomic number, is a fundamental characteristic that defines the element itself.
For instance, argon, an inert gas essential in many industrial applications, possesses 18 protons in its nucleus. This unique proton count not only establishes argon’s identity but also governs its chemical behavior and the properties that make it so valuable.
Understanding atomic structure is paramount to comprehending the behavior of protons and their profound impact on elements. Protons, along with neutrons, constitute the nucleus and determine an atom’s atomic mass. The number of electrons, in turn, influences an element’s chemical reactivity and its ability to form bonds.
Thus, the connection between nuclear physics and atomic structure allows us to unravel the secrets of protons and appreciate their crucial role in shaping the world around us.
Argon’s Protons: A Detailed Analysis:
- Reiterate argon’s atomic number of 18 and its corresponding 18 protons.
- Elaborate on how the number of protons impacts argon’s chemical behavior and characteristics.
- Compare argon to other elements with different proton numbers to highlight its significance.
Argon’s Protons: A Detailed Analysis
Argon, with an atomic number of 18, houses 18 protons within its nucleus. These protons, the fundamental building blocks of atoms, play a pivotal role in shaping the element’s identity and its interactions.
Argon’s Protons and Chemical Behavior
The number of protons in an atom determines its elemental nature. In argon’s case, its 18 protons define its status as a noble gas. Noble gases are renowned for their inertness; they form few chemical bonds due to their stable electron configurations. This stability stems from the balance of protons and electrons, which create a neutral charge distribution.
Argon’s Protons in the Periodic Table
The periodic table serves as a roadmap for understanding the elements. Argon’s position in Group 18 reflects its noble gas properties. This classification further highlights the significance of proton count in organizing and classifying the elements based on their reactivity and physical properties.
Argon’s Comparison to Other Elements
Comparing argon to other elements with different proton numbers sheds light on its unique characteristics. For instance, sodium, with 11 protons, readily reacts with water, while chlorine, with 17 protons, exhibits high reactivity with metals. Argon’s 18 protons, however, render it chemically inactive in most circumstances.
In conclusion, the 18 protons in argon’s nucleus are fundamental to its identity. They define its noble gas properties, determine its chemical behavior, and contribute to its position in the periodic table. Understanding the role of protons in atomic structure and nuclear physics is critical for comprehending the diverse properties of elements and their interactions in the world around us.