Argon: A Detailed Look At Its Atomic Structure, Group Classification, And Electron Configuration
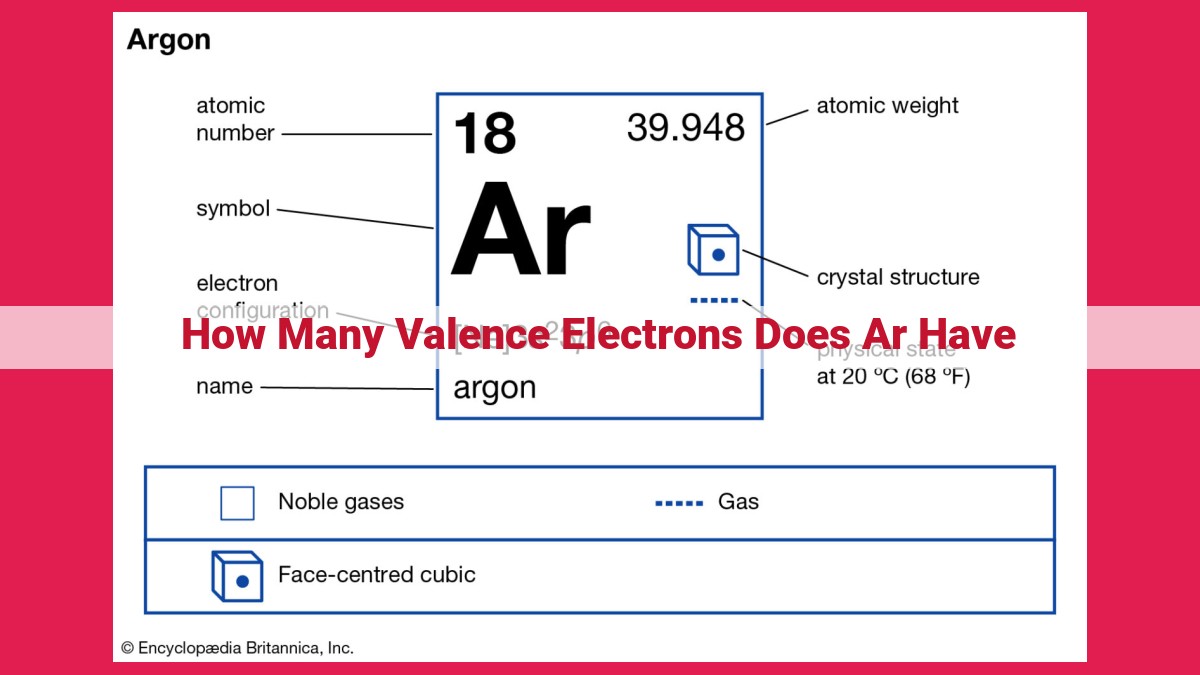
Argon (Ar) has an atomic number of 18, which corresponds to the number of protons in its nucleus. This atomic number places Ar in Group 18 (noble gases) of the periodic table. The group number of an element indicates the number of valence electrons it has, which are the electrons in the outermost energy level. For Ar, its group number (18) signifies that it has 8 valence electrons, giving it a stable electron configuration similar to noble gases.
Atomic Number: Unveiling the Identity of Elements
The microscopic world of atoms holds countless secrets, and one of the most fundamental is their atomic number. Imagine each element as a unique individual carrying a special “identity card” that distinguishes it from all others. This card, aptly named the atomic number, is a whole number that remains constant for each element. It’s the defining characteristic that shapes an element’s chemical identity and determines its place on the periodic table, the iconic arrangement of elements that guides chemists and scientists alike.
The atomic number of an element reveals the number of protons it contains, which reside in the heart of the atom, the nucleus. Protons are positively charged particles, and their number determines the element’s chemical identity. For instance, all atoms with one proton are hydrogen, regardless of any other subatomic particles they may possess. Similarly, all atoms with six protons are carbon, the building block of life. The atomic number, therefore, serves as the element’s fingerprint, distinguishing it from its chemical cousins.
Furthermore, the atomic number not only identifies an element but also governs its position on the periodic table. Elements are organized in ascending order of their atomic numbers, meaning that the element with atomic number 1 (hydrogen) sits on the far left, while the element with atomic number 118 (oganesson) resides on the far right. This arrangement isn’t arbitrary; it mirrors the periodic trends in chemical properties that make the periodic table such a powerful tool for understanding chemistry. Elements with similar chemical properties are grouped together, making it easier for scientists to predict the behavior of a particular element based on its position on the table.
In the realm of chemistry, the atomic number is a cornerstone concept, providing a foundation for comprehending the behavior and interactions of elements. It’s the key to unlocking the secrets of the microscopic world and understanding the diverse chemical tapestry that makes up our universe.
Group Number: Unlocking the Secrets of the Periodic Table
In the captivating world of chemistry, elements dance with elegance, each possessing a unique fingerprint that defines its behavior and properties. One of these crucial identifiers is the group number, an enigmatic number that holds the key to unlocking the secrets of element classification.
Imagine the periodic table as a grand ballroom, where elements gracefully waltz in their designated positions. The group number serves as a dance partner, guiding elements into their respective aisles. This number, like a cosmic address, reveals the number of valence electrons, the mischievous outlaws that reside in an element’s outermost energy level.
These valence electrons, with their unbound spirits, crave adventure and bonding. They embark on daring expeditions, seeking chemical partners to forge alliances and create new substances. The group number reflects this adventurous nature, offering a glimpse into an element’s potential for chemical mischief.
For instance, elements in Group 1 flaunt a single valence electron, making them ardent adventurers eager to partner up. In contrast, Group 18 elements, with their octet of valence electrons, are content souls, seeking only to maintain their stable status quo.
As we traverse the periodic table, the group number becomes an invaluable guide, revealing the chemical tendencies of each element. It’s a celestial compass, pointing us towards the elements most likely to form ionic bonds, covalent bonds, or even prefer solitude.
So, when faced with the enigma of the periodic table, remember the group number, the hidden dance partner that unlocks the secrets of element classification and paves the path to understanding their captivating chemistry.
Electronic Configuration: A Map of Electron Energy Levels
- Explain the concept of electronic configuration as the distribution of electrons in energy levels.
- Discuss the use of subshells and orbitals to describe electron arrangements.
Electronic Configuration: A Map of Electron Energy Levels
Imagine your atom as a bustling city, with electrons zipping around like commuters. The distribution of these electrons in different energy levels is known as its electronic configuration. It’s like a blueprint of your element, revealing its chemical behavior and place in the periodic table.
Each energy level, like a neighborhood, holds a specific number of electrons. These neighborhoods are called subshells, and they’re further divided into orbitals, which are the actual addresses where electrons reside. Orbitals are like tiny apartments, each accommodating a maximum of two electrons.
To describe an element’s electronic configuration, we use a shorthand notation that specifies the number of electrons in each subshell. For instance, an element with two electrons in the first subshell and six in the second would be written as 2, 6.
Delving deeper, we can picture the electrons as spinning around the nucleus like tiny planets. Each electron has a spin, represented by an up or down arrow. Electrons in the same orbital must have opposite spins, ensuring a harmonious coexistence in their atomic abode.
Understanding electronic configuration is crucial for grasping chemical bonding and reactivity. It’s like knowing the language your atoms speak, allowing you to predict how they’ll interact with each other and form molecules. It’s a roadmap to unraveling the mysteries of the chemical world.
Valence Electrons: The Matchmakers of Chemical Reactions
- Define valence electrons as the electrons in the outermost energy level.
- Explain their crucial role in chemical bonding and chemical reactivity.
Valence Electrons: The Matchmakers of Chemical Reactions
In the vast realm of chemistry, where elements dance and mingle, valence electrons emerge as the pivotal players, orchestrating the intricate choreography of chemical reactions. These electrons, like nimble matchmakers, navigate the delicate balance of atomic interactions, determining the destiny of molecules.
Valence electrons reside in the outermost energy level of an atom, like eager participants waiting to join the cosmic ballet. Their number, a crucial determinant of an element’s chemical identity, governs its ability to form bonds with other atoms.
In their matchmaking endeavors, valence electrons follow a simple yet profound rule: they strive to achieve a stable configuration, mirroring the inert stability of noble gases. This noble gas configuration, with a full complement of valence electrons, represents the epitome of electronic serenity.
When valence electrons embark on their bonding adventures, they may either be shared or transferred to another atom. In covalent bonds, valence electrons unite their forces, forming a harmonious partnership between atoms. In ionic bonds, valence electrons flee their atomic home, creating positively and negatively charged ions that dance in an electrostatic embrace.
The waltz of valence electrons dictates the reactivity of an element, which is why elements with similar numbers of valence electrons often share similar chemical properties. For instance, the alkali metals, with a solitary valence electron, readily donate it, forming strong bonds with electronegative elements like chlorine. Halogens, with seven valence electrons, eagerly accept an electron, forging covalent bonds with metals to achieve electronic equilibrium.
So, next time you witness a chemical reaction, remember the unseen dance of valence electrons. They are the architects of molecular harmony, the matchmakers of our chemical world. Understanding their role is like unlocking a secret code, granting us a deeper appreciation for the intricate symphony of chemical interactions.
The Periodic Table: A Window into the Patterns of Elements
Prepare yourself to embark on a captivating voyage through the wondrous realm of the periodic table – a masterpiece of scientific brilliance that unveils the secrets of the universe’s building blocks: the elements. Imagine a cosmic tapestry, woven together by the intricate dance of subatomic particles, with every element occupying a unique position on this celestial canvas.
Now, fix your gaze upon this extraordinary table, its rows and columns pulsating with the rhythms of atomic structure. Each element is defined by its atomic number, a numerical passport that reveals its elemental identity. This numerical fingerprint, like a cosmic calling card, determines an element’s position on the table and bestows upon it a set of defining characteristics that set it apart from all others.
As you traverse this periodic landscape, you’ll encounter fascinating patterns that emerge, like celestial constellations. Elements grouped together in vertical columns, known as groups, share a common trait: the number of valence electrons. These electrons, the social butterflies of the atomic world, determine an element’s chemical reactivity and its eagerness to form bonds with other elements.
But the periodic table’s allure doesn’t end there. Across the table’s horizontal rows, known as periods, another intriguing pattern unfolds. Elements within a period share the same number of electron shells, those ethereal energy levels that house electrons. As you move from left to right across a period, the number of electrons steadily increases, shaping the element’s properties and behavior.
## Electron Affinity and Electronegativity: The Tug-of-War of Attraction
As we delve deeper into the periodic table’s mysteries, we encounter two captivating properties: electron affinity and electronegativity. Electron affinity measures an element’s desire to acquire electrons, its willingness to embrace a negatively charged embrace. Electronegativity, on the other hand, captures an element’s possessive grip on its electrons, its reluctance to share its precious charges.
These two properties dance together across the periodic table, creating a mesmerizing choreography. As you move from left to right across a period, electron affinity generally increases, signifying a growing eagerness to welcome electrons. Simultaneously, electronegativity also rises, indicating a stronger hold on electrons.
## The Noble Gas Haven: A Sanctuary of Stability
At the far right-hand side of the periodic table, we encounter the noble gases, the aristocrats of the element world. These elements, with their inert nature, have attained a state of electronic bliss – a noble gas configuration. This configuration bestows upon them an enviable stability, a reluctance to engage in chemical reactions.
The noble gases serve as celestial beacons, guiding other elements in their quest for stability. Many elements strive to mimic the noble gas configuration by gaining or losing electrons, forming chemical bonds to achieve this coveted state of equilibrium.
The periodic table is not merely a static arrangement of elements; it’s a dynamic tapestry woven with the threads of atomic structure and chemical behavior. Through its patterns and trends, we gain invaluable insights into the nature of matter and the intricate dance of subatomic particles. Whether you’re a seasoned chemist or a curious explorer of the universe, the periodic table awaits your exploration, ready to unveil its secrets and ignite your imagination.
Noble Gas Configuration: The Epitome of Electron Stability
In the captivating realm of chemistry, noble gas configuration reigns supreme as the coveted state of atomic stability. Noble gases, like the noble knights of the periodic table, possess an aura of completeness and an uncanny ability to remain aloof from chemical interactions. Their secret lies in their electron configuration, a blueprint that reveals the arrangement of electrons within their atomic structure.
Noble gases occupy the far right column of the periodic table, their electron configurations adorned with a special trait: a full outer energy level. This arrangement bestows upon them an unmatched stability, rendering them chemically unreactive and content in their solitary existence.
Like a wise king surrounded by a loyal court, the nucleus of a noble gas atom is shielded by its valence electrons, the electrons that reside in its outermost energy level. These valence electrons are the key players in chemical bonding, the process by which atoms connect to form molecules. However, in noble gases, they remain steadfastly inert, unwilling to participate in the chemical dance.
This reluctance stems from the fact that noble gases already possess a full complement of valence electrons. They have no desire to acquire more or surrender what they have, maintaining a state of perfect balance. This stability is akin to a puzzle with all its pieces perfectly in place, leaving no room for further rearrangements.
In contrast, other elements often embark on a quest to achieve this noble gas configuration. Some may shed their excess valence electrons, like a king casting off his heavy crown, to attain the coveted stability of a noble gas. Others may don the mantle of additional valence electrons, like a queen adorning herself with jewels, to complete their electron configuration and reach the pinnacle of chemical contentment.
Understanding noble gas configuration is not merely an academic exercise; it holds profound implications for understanding the chemical world around us. It provides a framework for predicting chemical reactivity, unraveling the mysteries of periodic trends, and unlocking the secrets of molecular interactions. So, as we delve deeper into the fascinating realm of chemistry, let us never forget the profound significance of noble gas configuration, the epitome of electron stability.