Antimony: A Unique Metalloid With Versatile Bonding Capabilities Due To Five Valence Electrons
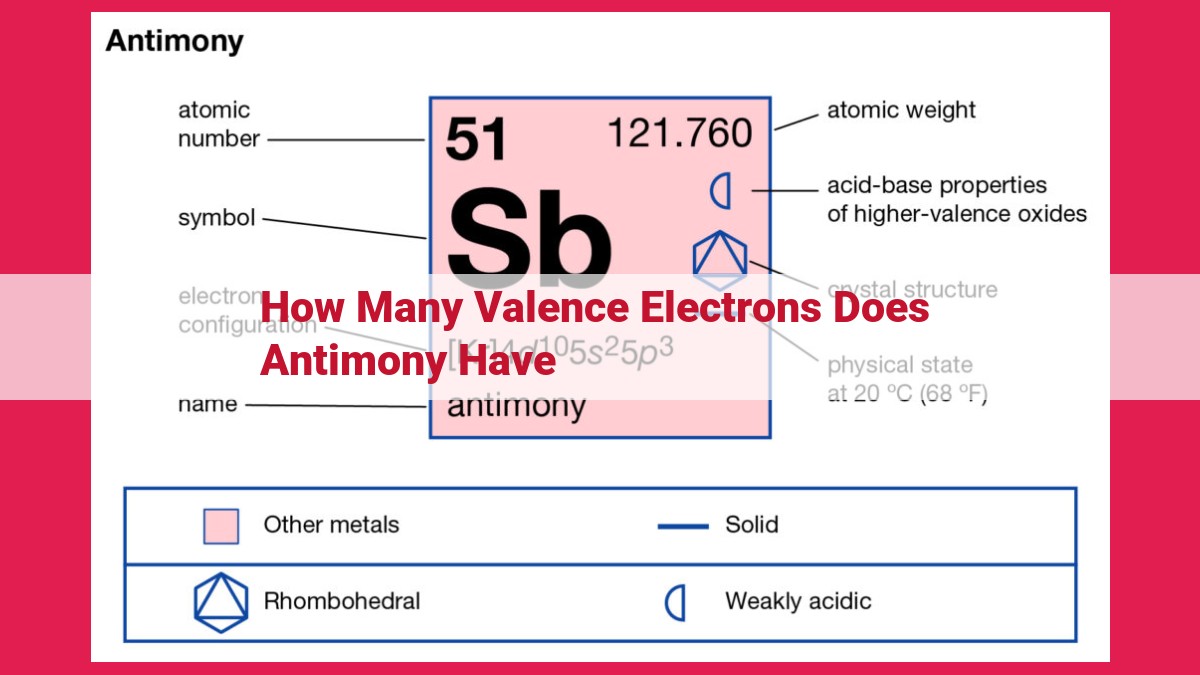
Antimony, a metalloid element with the symbol Sb and atomic number 51, has 5 valence electrons. Its electron configuration, [Kr]4d¹⁰5s²5p³, indicates that the outermost shell contains three valence electrons. Valence electrons play a crucial role in determining an element’s chemical properties, including its ability to form bonds with other atoms. In antimony’s case, its five valence electrons allow it to exhibit various chemical bonding behaviors, influencing its chemical reactions and applications in various fields.
Antimony’s Valence Electrons: Unveiling the Gateway to Chemical Reactions
Antimony, an enigmatic element nestled in the heart of the periodic table, holds a secret that unlocks the door to its fascinating chemical behavior: its valence electrons. These electrons, like tiny messengers, dance around the antimony atom, dictating its ability to form bonds, shape molecules, and interact with the world around it.
In this blog post, we’ll embark on a captivating journey to unravel the mystery of antimony’s valence electrons. We’ll explore their definition, delve into their significance, and witness their crucial role in shaping antimony’s chemical destiny.
Understanding Valence Electrons
Valence electrons reside in the outermost energy level of an atom, eagerly awaiting the opportunity to engage in chemical bonding. They are the most influential electrons, determining an element’s chemical properties and reactivity.
Antimony: The Element
Antimony, a metalloid with a unique blend of metallic and non-metallic characteristics, sits comfortably in Group 15 of the periodic table. Its symbol, Sb, atomic number 51, and notable properties have captivated scientists for centuries.
Atomic Number and Electron Configuration
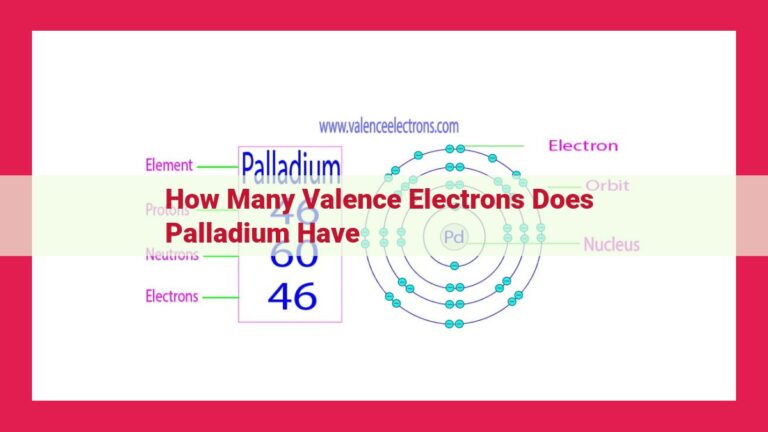
The atomic number of an element reveals the number of protons in its nucleus and determines its position on the periodic table. Antimony’s atomic number, 51, signifies the presence of 51 protons in its nucleus.
Electron configuration, a blueprint of an atom’s electron arrangement, provides valuable insights into its chemical behavior. Antimony’s electron configuration, [Kr] 4d¹⁰ 5s² 5p³, reveals the presence of three valence electrons in its outermost energy level.
Valence Electrons in Antimony
The calculation of valence electrons is straightforward: simply count the electrons in the outermost energy level. Antimony, with its three valence electrons, is eager to participate in chemical reactions by gaining, losing, or sharing these electrons.
Significance of Antimony’s Valence Electrons
Antimony’s valence electrons play a pivotal role in forming chemical bonds. They determine the element’s ability to react with other atoms and molecules, shaping its chemical bonding capabilities.
Related Concepts
Chemical bonding, the dance of atoms and molecules, is intimately linked to valence electrons. The periodic table, a treasure map of elements, provides clues about an element’s valence electrons based on its position within its group.
Antimony’s valence electrons are not merely abstract concepts but the key to understanding its chemical behavior. By unraveling the secrets of these tiny messengers, we gain a deeper appreciation for the intricate tapestry of chemical interactions that shape our world.
Understanding Valence Electrons
In the vast realm of chemistry, valance electrons play a pivotal role in shaping the behavior and properties of elements. These are the electrons that reside in the outermost energy level of an atom, and they are pivotal in determining how elements interact with one another.
Think of an atom as a miniature solar system, with a nucleus at the center and electrons orbiting around it like planets. The innermost energy level is closest to the nucleus, followed by the outermost energy level. Valence electrons are the “wanderers” of the atom, as they are not firmly attached to the nucleus and have a higher energy state.
The number and arrangement of valence electrons significantly influence an element’s chemical properties. These outermost electrons are the most involved in chemical reactions, determining how an element will bond with other elements. For instance, elements with one valence electron tend to be highly reactive, readily forming bonds to achieve a stable configuration. In contrast, elements with a full valence shell exhibit lower reactivity.
The chemistry that governs our world is largely driven by the interplay of valence electrons. These tiny particles are the key to understanding how elements combine to form molecules and compounds, shaping the intricate tapestry of matter that surrounds us.
Antimony: The Enigmatic Metalloid
Unveiling the Mysterious World of Antimony
In the realm of chemistry, elements fascinate us with their unique characteristics and captivating stories. One such element is antimony, a metalloid that bridges the gap between metals and nonmetals. Let’s explore its captivating world, starting with its fundamental qualities.
Classification: A Versatile Metalloid
Antimony belongs to the enigmatic group of elements known as metalloids, substances that exhibit both metallic and nonmetallic properties. This duality endows antimony with an unusual combination of traits, making it an essential component in various technological applications.
Symbol, Atomic Number, and General Properties
The chemical symbol for antimony is Sb, and its atomic number is 51. This means that each antimony atom possesses 51 protons and 51 electrons, arranged in a specific configuration that determines its chemical behavior. Antimony’s silvery-white appearance, brittleness, and relatively high electrical conductivity are just a few of its distinctive general properties.
Delving into the Realm of Atomic Number and Electron Configuration
In the vast realm of chemistry, the concept of atomic number holds paramount importance. It’s the defining characteristic of an element, a fundamental fingerprint that distinguishes it from all others. For every element on the periodic table, the atomic number is a unique integer that represents the number of protons residing within its nucleus.
Electrons, on the other hand, are the tiny, negatively charged particles that orbit the nucleus. Understanding electron configuration is crucial because it governs the chemical properties of an element. Electron configuration refers to the arrangement of electrons in the various energy shells around the nucleus.
The electrons occupying the outermost shell, known as the valence electrons, play a vital role in determining an element’s reactivity and its ability to form chemical bonds. The number of valence electrons is directly related to the element’s position on the periodic table. In the case of antimony, an element belonging to Group 15, it boasts five valence electrons, rendering it a versatile player in the world of chemical reactions.
Valence Electrons in Antimony: Unlocking the Chemical Secrets of a Metalloid
In the realm of chemistry, understanding the behavior of elements is crucial for unraveling the mysteries of chemical reactions. One key aspect that governs an element’s reactivity is its valence electrons. Let’s delve into the captivating world of antimony, a fascinating metalloid, and uncover the significance of its valence electrons.
Calculating Antimony’s Valence Electrons
The number of valence electrons in an element is directly related to its atomic number. Antimony, with an atomic number of 51, has 51 electrons orbiting its nucleus. According to the periodic table, elements in Group 15, like antimony, have five valence electrons. This means that antimony has five electrons in its outermost energy level, ready to participate in chemical bonding.
Electron Configuration and Valence Electrons
To further understand antimony’s valence electrons, we need to examine its electron configuration. The electron configuration represents the distribution of electrons in different energy levels around the nucleus. Antimony’s electron configuration is:
1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁶ 4d¹⁰ 5s² 5p³
The five electrons in the outermost energy level, denoted by 5s² 5p³, are antimony’s valence electrons. These electrons occupy the highest energy orbitals and are responsible for antimony’s chemical reactivity.
Significance of Antimony’s Valence Electrons
Valence electrons play a pivotal role in determining an element’s chemical properties. They determine the types of bonds an element can form and its ability to react with other elements. Antimony’s five valence electrons allow it to participate in various chemical reactions, forming bonds with nonmetals like oxygen, halogens, and sulfur.
For instance, antimony can form covalent bonds with oxygen to create antimony trioxide (Sb₂O₃), a compound used in glass and ceramic production. It can also bond with chlorine to form antimony trichloride (SbCl₃), a colorless liquid used as a catalyst in organic chemistry.
Unraveling the mystery of antimony’s valence electrons is essential for comprehending its chemical behavior. With five valence electrons, antimony possesses the ability to form diverse bonds, making it a versatile element in various chemical processes. From glass production to catalysis, antimony’s valence electrons play a crucial role in shaping the world around us. Understanding their significance deepens our appreciation for the intricacies of chemistry and the fascinating world of elements.
Valence Electrons and the **Chemical Bonding Capabilities of Antimony**
Valence electrons play a pivotal role in the chemical interactions of elements. For antimony, a fascinating metalloid, these electrons determine its ability to form bonds with other atoms.
Antimony’s atomic number, 51, indicates that it has 51 electrons, including its valence electrons. These valence electrons are located in the outermost shell of the atom and dictate antimony’s chemical behavior.
With five valence electrons, antimony can participate in a variety of chemical bonding arrangements. It commonly forms covalent bonds, sharing electrons with other atoms to achieve a stable configuration. For instance, in the molecule antimony trichloride (SbCl₃), each chlorine atom shares one valence electron with antimony, resulting in a stable compound.
Antimony’s triple bonding capability is particularly noteworthy. By utilizing its three lone pairs of valence electrons, antimony can form three covalent bonds with other atoms. This ability is evident in molecules like antimony trioxide (Sb₂O₃) and antimony sulfide (Sb₂S₃).
Furthermore, antimony’s valence electrons enable it to participate in ionic bonding as well. By losing or gaining electrons, antimony can acquire a stable charge. In ionic compounds like potassium antimonate (K₂SbO₃), antimony assumes a positive charge (+3) by losing three valence electrons.
In summary, antimony’s valence electrons are essential for understanding its chemical bonding capabilities. These electrons dictate the number and type of bonds antimony can form, enabling it to participate in a diverse range of chemical reactions.
Related Concepts
- Brief discussion of chemical bonding and its connection to valence electrons
- Mention of the periodic table and the significance of antimony’s position within Group 15
Antimony’s Valence Electrons: Unveiling the Secrets of a Versatile Metalloid
Delve into the fascinating world of antimony, a unique metalloid that plays a crucial role in our technological advancements. In this article, we’ll explore the significance of antimony’s valence electrons, uncovering their pivotal influence on its chemical properties and bonding capabilities.
Understanding Valence Electrons
Valence electrons, the outermost electrons in an atom, are like the sociable extroverts of the atomic realm. They determine how an element interacts with others, forming the foundation of chemical bonding. Antimony, with its intriguing position in the periodic table, has a captivating story to tell about its valence electrons.
Antimony: A Metalloid with Diverse Properties
Antimony, a metalloid, straddles the boundary between metals and nonmetals. This duality grants it an array of properties, from its silvery-blue luster to its semi-conducting abilities. Understanding antimony’s valence electrons is key to unraveling the secrets of its versatility.
Atomic Number and Electron Configuration
Every element has a unique atomic number, which dictates the number of protons and electrons in its atoms. Antimony’s atomic number is 51, indicating the presence of 51 electrons. The arrangement of these electrons, known as the electron configuration, reveals the number of valence electrons.
Valence Electrons in Antimony
Antimony’s atomic number of 51 means it has 51 electrons. Its electron configuration, written as [Kr] 4d¹⁰5s²5p³, shows that antimony has five valence electrons in the outermost shell.
Significance of Antimony’s Valence Electrons
These five valence electrons play a pivotal role in antimony’s chemistry. They enable antimony to form bonds with a variety of elements, contributing to its diverse applications in electronics, alloys, and flame retardants. Antimony’s valence electrons are the driving force behind its ability to conduct electricity and resist heat.
Related Concepts: Bonding and the Periodic Table
Chemical bonding, the process by which atoms combine to form molecules and compounds, is closely intertwined with valence electrons. Antimony’s valence electrons determine its bonding capabilities, allowing it to form covalent, ionic, and metallic bonds.
Antimony’s position in Group 15 of the periodic table also sheds light on its valence electrons. Group 15 elements, also known as pnictogens, typically have five valence electrons, giving them similar chemical properties. By understanding antimony’s position in the periodic table, we gain valuable insights into its valence electrons and their implications for its behavior.