Unveiling The Optimal Ph Range For Amylase: A Guide To Maximizing Enzyme Efficiency In Various Industries
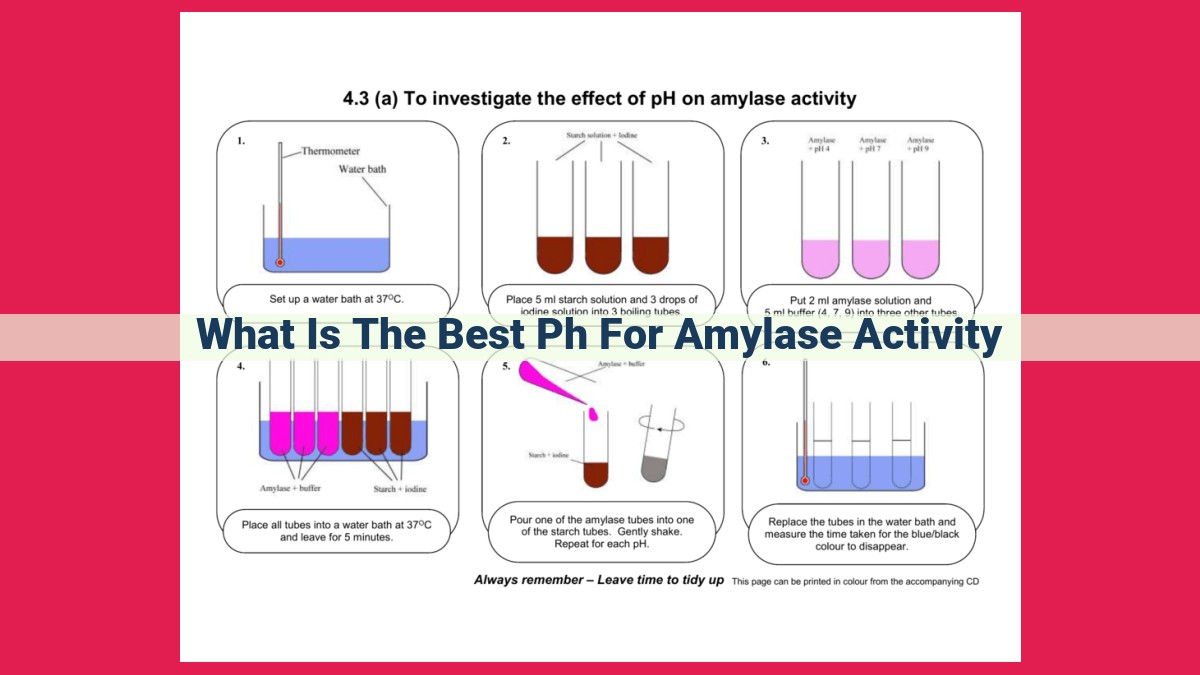
Amylase, a crucial enzyme in industries like food processing and brewing, has an optimal pH range for activity. Understanding this range is vital for maximizing enzyme efficiency. The pH affects amylase’s conformation, substrate binding, and overall performance. Factors like temperature, substrate concentration, and enzyme quantity also influence activity. By selecting the appropriate pH, specific applications can benefit from enhanced amylase performance. Operating enzymes outside their optimal pH range can disrupt activity and compromise their efficiency.
Enzyme Activity and pH: The Key to Optimization
In the realm of enzymes, “little biochemical workhorses,” knowing their optimal pH is paramount. pH, a measure of acidity or alkalinity, plays a decisive role in enzyme activity. It dictates their molecular shape, charge distribution, and consequent ability to bind to substrates, the molecules they work on.
Just as Goldilocks sought the perfect porridge temperature, enzymes demand a specific pH “sweet spot” to function optimally. Deviating from this ideal pH can disrupt their structure like a fragile snowflake in a storm, hindering their ability to interact with substrates effectively. It’s like trying to fit a square peg into a round hole.
Optimizing enzyme activity through careful pH control is crucial for various industries that rely on these biological catalysts. Take amylase, for example, a versatile enzyme used in food processing, brewing, and starch production. Amylase’s optimal pH depends on its intended application. In baking, it’s tweaked to enhance dough development, while in brewing, it’s adjusted to optimize fermentation.
Understanding the pH-dependence of enzyme activity is not just academic whimsy. It’s a practical necessity for industries harnessing enzymes’ power. By choosing the appropriate pH, manufacturers can boost efficiency, maximize productivity, and ensure consistent product quality.
pH optimization is not a one-time affair. Monitoring and controlling pH are ongoing tasks, especially in large-scale industrial settings. Slight pH fluctuations can have cascading effects on enzyme activity and product yield. Regular pH checks and adjustments ensure that enzymes remain in their optimal environment, performing like well-oiled machines.
In closing, understanding the interplay between enzyme activity and pH is crucial for unlocking the full potential of these biological marvels. Optimizing pH creates an environment where enzymes can flourish, leading to efficient industrial processes and superior product quality.
Amylase: The Enzyme Behind Industrial Transformations
Deep in the world of enzymes, amylase stands out as a true culinary virtuoso, capable of transforming starch into a symphony of sweet and complex flavors. This remarkable enzyme is an indispensable player in countless industries, including food processing, brewing, and starch production.
Imagine the aroma of freshly baked bread wafting through the air. That irresistible crust and fluffy interior are a testament to the magic of amylase. This enzyme breaks down starch, the main carbohydrate in wheat, into sugars that yeast can feast upon, leading to the rise and golden-brown color of our beloved bread.
In the world of brewing, amylase’s role is equally crucial. It unlocks the sugars hidden within barley, providing the essential fuel for fermentation. The resulting beer delights us with its enchanting flavors and aromas.
The Starch Wizard in Starch Production
Beyond food and beverages, amylase plays a pivotal role in the production of starch, a versatile ingredient used in a wide range of industries. From papermaking to textiles, starch adds strength, smoothness, and stability to various products. Amylase breaks down starch into smaller molecules, enabling its extraction and modification to meet specific industrial needs.
pH: The Key to Amylase’s Success
Just as a chef carefully adjusts the seasoning to enhance a dish’s flavor, pH plays a critical role in optimizing amylase’s performance. Each enzyme has an optimal pH range where it functions most efficiently. For amylase, the sweet spot lies between 6.0 and 7.0.
Operating within this optimal pH range ensures that amylase’s molecular structure remains intact, allowing it to bind to starch molecules and catalyze the breakdown process with precision. Deviations from this ideal pH can compromise the enzyme’s activity, leading to reduced efficiency or even complete enzyme disruption.
Precision pH Control: A Recipe for Industrial Excellence
In the industrial arena, precise pH control is paramount for maximizing amylase’s productivity and ensuring the desired product quality. By carefully monitoring and adjusting pH levels, food processors, brewers, and starch manufacturers can unlock the full potential of this remarkable enzyme.
By understanding the intricate relationship between amylase and pH, we can harness its power to create delicious foods, refreshing beverages, and essential industrial materials. As we continue to delve into the enchanting world of enzymes, the future holds endless possibilities for harnessing their transformative abilities.
The Sweet Spot for Amylase Activity: Exploring the pH Range
In the realm of enzymes, understanding the optimal pH for their activity is crucial for maximizing their potential. Amylase, a starch-digesting enzyme with widespread industrial applications, is no exception.
Amylase’s pH Range: A Balancing Act
Like a delicate dance, amylase’s activity is exquisitely sensitive to pH. It performs optimally within a narrow range of pH values, typically between 5.5 and 7.0. Below or above this range, its catalytic prowess diminishes. This pH dependence stems from the enzyme’s intricate structure and the delicate balance of charges that enable it to interact effectively with its substrate, starch.
pH and Enzyme Conformation: A Structural Dilemma
pH has a profound influence on amylase’s conformation, the three-dimensional shape that dictates its functionality. At the optimal pH, amylase’s structure is stabilized, allowing it to bind to and hydrolyze starch molecules with precision. Deviations from this optimal pH can disrupt the enzyme’s conformation, impairing its ability to interact with its substrate and reducing its catalytic efficiency.
Significance of pH Optimization
The importance of optimizing pH for amylase’s activity cannot be overstated. In industrial settings, where amylase is employed for various purposes, maintaining the appropriate pH can significantly enhance its performance and productivity.
For instance, in the food industry, amylase’s ability to break down starch into fermentable sugars is essential for the production of high-quality baked goods, beverages, and other products. Optimizing pH ensures efficient starch conversion, leading to optimal product quality and taste.
Understanding the pH dependence of amylase activity is fundamental for harnessing its full potential in industrial applications. By maintaining the optimal pH, we can maximize its catalytic efficiency and ensure its optimal performance. This precision control not only enhances productivity but also contributes to the development of high-quality products that meet consumer expectations.
pH’s Impact on Amylase’s Structure and Performance
The pH of the environment plays a crucial role in determining the performance of amylase, an enzyme responsible for breaking down starch into simpler sugars. Understanding the relationship between pH and amylase activity is essential for optimizing enzyme utilization in various industrial applications.
pH affects the stability and conformation of the amylase protein. Each enzyme has an optimal pH range where its structure and catalytic activity are maximized. Deviations from this optimal pH can lead to changes in the enzyme’s conformation, affecting its ability to bind to and interact with its substrate (starch).
At acidic pH, the positively charged amino acid residues on the amylase surface become protonated, reducing their electrostatic interactions with the negatively charged starch molecules. This impairs the enzyme’s ability to bind to its substrate, reducing its catalytic activity.
Conversely, at alkaline pH, the positively charged amino acid residues become deprotonated, resulting in a net decrease in the enzyme’s positive charge. This can lead to electrostatic repulsion between the enzyme and the negatively charged starch, further hindering their interaction.
Additionally, extreme pH conditions can denature the enzyme protein, causing it to lose its native structure and catalytic activity. This process is irreversible and significantly reduces the enzyme’s effectiveness.
Optimizing pH for specific applications is crucial to ensure maximal amylase activity. For example, in the brewing industry, amylase enzymes are used to break down starch into fermentable sugars, which are utilized by yeast to produce alcohol. The pH of the brewing mash is carefully controlled to maintain the optimal conditions for amylase activity.
Understanding the pH-dependence of amylase and other enzymes is essential for their efficient utilization in various industrial processes. By carefully controlling the pH, researchers and industry professionals can optimize enzyme performance, resulting in increased efficiency and productivity.
Temperature, Substrate, and Enzyme: The Balancing Act
Every enzyme, including amylase, operates within specific constraints beyond pH. Temperature plays a crucial role in enzyme activity. Each enzyme has an optimal temperature range where it exhibits maximum reactivity. Beyond this range, enzyme activity rapidly declines.
Substrate concentration also influences amylase’s performance. When substrate concentration is low, the enzyme’s active sites are not fully occupied, resulting in lower catalytic efficiency. On the other hand, excessively high substrate concentrations can lead to enzyme saturation, where all active sites are occupied but the reaction rate remains constant.
Enzyme concentration is another factor that affects amylase activity. Increasing enzyme concentration will generally increase the reaction rate, as more enzyme molecules are available to catalyze the reaction. However, this relationship is not always linear, and at very high enzyme concentrations, the reaction rate may plateau due to factors such as substrate depletion or product inhibition.
Understanding the combined effects of pH, temperature, substrate concentration, and enzyme concentration can help optimize amylase activity for specific industrial applications. By tailoring these parameters, manufacturers can maximize enzyme performance, reduce costs, and ensure efficient production processes.
Amylase: A Versatile Catalyst in Industrial Applications
Amylase, an enzyme renowned for its remarkable ability to break down starch, plays a pivotal role in various industries, transforming raw materials into valuable products.
Food Processing:
In the realm of food processing, amylase shines as an indispensable ingredient. It helps tenderize baked goods, resulting in soft, fluffy textures. In the production of syrups and sauces, amylase converts starch into sugars, enhancing their sweetness and consistency.
Brewing:
Within the brewing industry, amylase is a key player in the conversion of starch into fermentable sugars. This crucial step in the brewing process allows yeast to transform these sugars into alcohol, giving beer its intoxicating character.
Starch Production:
Amylase holds immense significance in the starch production industry. It enables the efficient breakdown of starch into simpler carbohydrates, facilitating the extraction and purification of glucose and other valuable starch derivatives.
Specific Applications Across Industries:
- Food Additives: Amylase finds wide application as a food additive, improving texture and shelf life of products such as breads, pastries, and sauces.
- Brewing: In the brewing process, amylase plays a vital role in the production of wort, the fermentable liquid that forms the foundation of beer.
- Alcohol Production: Amylase is employed in the fermentation of grains, enabling the conversion of starch into sugars for subsequent alcohol production.
- Textile Industry: Amylase is utilized in the desizing process, removing starch from textiles to improve their appearance and feel.
- Paper Industry: Amylase is applied in paper manufacturing to enhance the strength and quality of paper by modifying its starch content.
pH Precision: Unleashing the Power of Amylase for Specific Applications
The optimal pH for an enzyme can significantly influence its performance. This is especially true for amylase, an enzyme with a wide range of applications in industries such as food processing, brewing, and starch production.
Tailoring Amylase Activity to Desired Outcomes
The pH of a solution determines the ionization state of amino acids within the enzyme, potentially altering its structure and function. By carefully selecting the appropriate pH, manufacturers can fine-tune amylase activity to suit specific applications.
For example, in the food processing industry, amylase is used to break down starches into smaller molecules. Acidic conditions (pH 4-5) promote amylase activity, making it ideal for applications such as tenderizing meat and preparing acidic foods. Conversely, in the brewing industry, amylase is used to convert starch into fermentable sugars. A higher pH (pH 5-6) is preferred, as it supports the optimal activity of the enzyme in the brewing process.
Engineering Enzymes for Enhanced Performance
Advances in biotechnology have allowed for the engineering of amylases with altered pH profiles. This enables researchers to create enzymes that are active under specific pH conditions. For instance, pH-stable amylases have been developed for use in industrial processes that require enzymes to withstand extreme pH environments.
Ensuring Enzyme Stability
Operating enzymes outside their optimal pH range can lead to a decrease in activity, reduced stability, and even enzyme denaturation. It is crucial to maintain a consistent pH within the acceptable range to ensure optimal amylase performance.
Monitoring and controlling pH is essential in industrial settings where enzymes are used. Sensors can be employed to measure pH in real-time, allowing for adjustments to be made promptly. This ensures that the enzyme remains active and operates at its peak efficiency.
By optimizing the pH for specific applications, manufacturers can unlock the full potential of amylase. This knowledge enables them to tailor enzyme performance, enhance product quality, and maximize the efficiency of industrial processes.
Consequences of pH Drift: The Dangers of Enzyme Disruption
Imagine a delicate ballerina twirling gracefully on stage. If the music suddenly speeds up or slows down, her performance would falter. Similarly, enzymes, the tiny protein machines that drive countless biological processes, require precise conditions to function optimally. One of the most crucial factors is pH.
When pH drifts away from the enzyme’s optimum, its structure and activity can be compromised. Like a sturdy bridge weakened by erosion, enzymes can become less stable and flexible, hindering their ability to bind with substrates and catalyze reactions.
The consequences of pH drift can be severe. In industrial settings, where enzymes are used in large-scale processes, even a slight pH deviation can disrupt productivity. Processes may slow down, product yields may drop, and energy consumption may increase.
For example, in the food industry, amylase, an enzyme that breaks down starch, is crucial for producing many products, including bread and beer. If the pH of the dough or the beer mash deviates from the enzyme’s optimum, the flavor, texture, and quality of the final products can be compromised.
In medical applications, pH drift can also have serious repercussions. Many diagnostic tests rely on enzymes to detect the presence of specific substances or diseases. If the pH of the test sample is not carefully controlled, false results may occur, potentially leading to misdiagnoses and incorrect treatment.
Therefore, maintaining the optimal pH for enzymes is essential for their proper function and the efficient operation of both industrial and medical processes. Careful monitoring and adjustment of pH levels are crucial to prevent enzyme disruption and ensure the integrity of the reactions they facilitate.
Looking Ahead: The Future of pH-Enzyme Research
In our quest to harness the power of enzymes, understanding their intricate relationship with pH is crucial. As we delve into the future of pH-enzyme research, exciting possibilities await.
Expanding Optimal pH Knowledge:
Researchers are embarking on a journey to explore the pH optima of understudied enzymes. This endeavor will unveil new enzymes with remarkable catalytic capabilities at specific pH values, broadening our enzymatic toolbox.
Precision pH Control:
The development of pH-responsive materials and sensors holds immense promise. These innovations will enable precise control of pH in enzymatic reactions, allowing for fine-tuning of enzyme activity and optimization for specific applications.
pH-Stable Enzymes:
Scientists are engineering pH-stable enzymes that can withstand extreme pH conditions. This will revolutionize industries that rely on enzymes in harsh environments, reducing the need for pH adjustment and expanding the scope of enzymatic applications.
pH-Responsive Enzyme Delivery Systems:
Novel pH-responsive delivery systems are being designed to encapsulate enzymes and release them only when the optimal pH is achieved. This targeted approach will enhance enzyme specificity, reduce side reactions, and improve therapeutic outcomes in the medical field.
Computational Modeling and Data Analysis:
Computational modeling and big data analysis are becoming powerful tools in predicting enzyme activity based on pH. These techniques will accelerate the discovery and design of new enzymes with tailored pH profiles, significantly reducing the time and cost of experimental research.
By unraveling the intricacies of pH-enzyme interactions, we unlock the potential for transformative advancements in various fields. From optimizing industrial processes to revolutionizing healthcare, the future of pH-enzyme research holds boundless opportunities.