Understanding Amino Acids: Essential Functional Groups And Their Roles
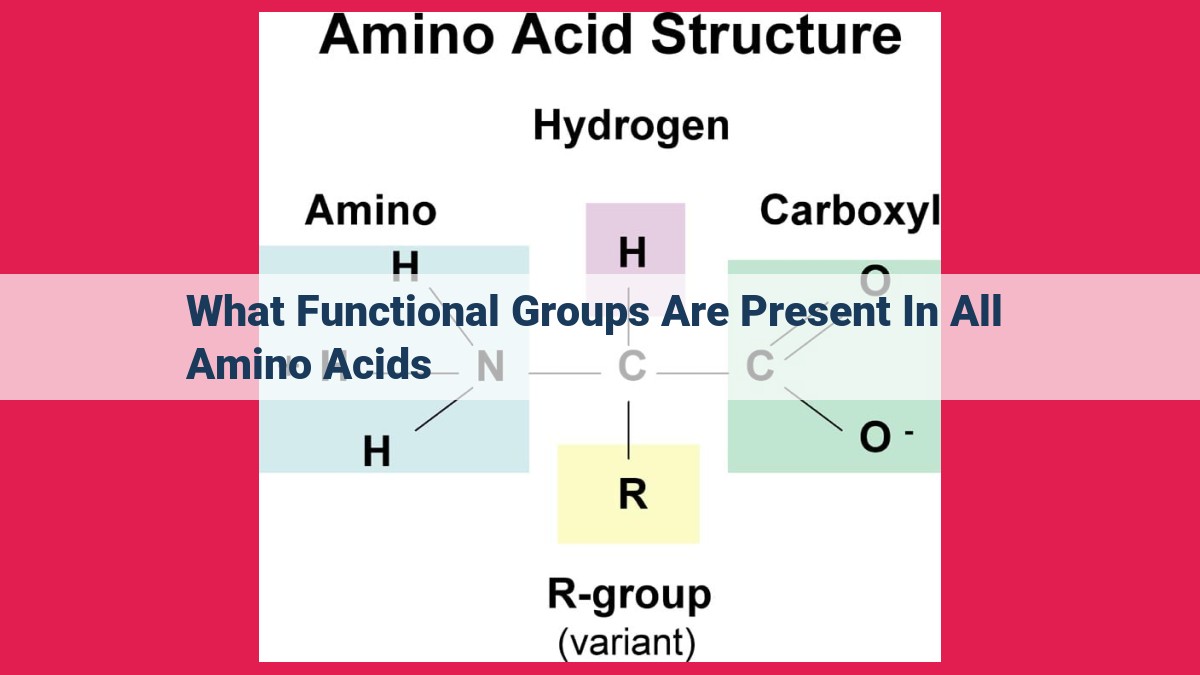
All amino acids possess two essential functional groups: the carboxyl group (-COOH) and the amino group (-NH2). The carboxyl group allows amino acids to act as acids and form carboxylate ions (-COO-), while the amino group enables them to function as bases and form ammonium ions (-NH3+). Additionally, amino acids have a variable side chain (R group) that differs based on the amino acid, influencing its chemical properties and biological function.
Amino Acids: The Building Blocks of Proteins
In the realm of biology, amino acids hold a central role as the fundamental building blocks of proteins. These intricate molecules form the structural framework of our bodies and orchestrate the biochemical symphony of life. Essential for life, amino acids serve as the foundation for every living organism.
Each amino acid is a unique entity, characterized by a unique side chain or R group. This side chain, like a fingerprint, determines the specific properties and functions of each amino acid. Some side chains are hydrophobic, preferring the company of other hydrophobic molecules, while others are hydrophilic, seeking the embrace of water.
Central to the structure of every amino acid lies the carboxyl group (-COOH), a region of acidity, and the amino group (-NH2), a region of basicity. These functional groups, like a molecular tug-of-war, form the basis of amino acid interactions. The dance between the carboxyl and amino groups creates the backbone of proteins, linking amino acids into intricate chains.
The diversity of side chains among amino acids is astounding, creating a vast repertoire of molecular possibilities. These side chains endow amino acids with an array of properties, including solubility, charge, and reactivity. They shape the molecular landscape of amino acids, influencing their interactions with other molecules and dictating their biological roles.
Explain their importance and their unique functional groups
Amino Acids: The Building Blocks of Life and Their Unique Functional Groups
Imagine yourself as a skilled builder, tasked with constructing a magnificent edifice. Just as bricks are to buildings, amino acids are the fundamental units that form the intricate structures of all life on Earth. These remarkable molecules play an indispensable role in every aspect of our biological existence, from the DNA that governs our genetic makeup to the proteins that power our bodies.
Amino acids are extraordinary not only for their role as building blocks but also for their unique functional groups. Imagine these functional groups as tiny chemical flags that give each amino acid its distinctive properties. The carboxyl group (-COOH), the first of these flags, is a sourpuss of a molecule. It’s always ready to donate a hydrogen ion, making amino acids slightly acidic.
But wait, there’s more! The amino group (-NH2) is the complete opposite of the carboxyl group. It’s a cheerful optimist, eager to accept hydrogen ions, lending amino acids a slightly basic character. These two functional groups create a harmonious balance within amino acids, allowing them to play dual roles as both acids and bases.
But the story doesn’t end there. Each amino acid also boasts a special side chain, denoted as R. This is where the real fun begins! Side chains vary widely in their structure and properties, giving rise to the diverse array of amino acids found in nature. Some side chains are polar, meaning they love to interact with water, while others are hydrophobic, shunning water like the plague.
The nature of the side chain profoundly influences the behavior and function of amino acids. Polar side chains tend to be found on the surface of proteins, interacting with the watery environment outside the cell. Hydrophobic side chains, on the other hand, prefer to hide away inside proteins, creating a hydrophobic core that protects the protein from water damage.
It’s through these interplay of functional groups that amino acids orchestrate the intricate dance of life. Their acidity, basicity, and side chain properties work in unison to determine the structure, function, and interactions of proteins, the workhorses of our cells. Without these remarkable molecules, life as we know it simply wouldn’t be possible.
Describe the structure and chemical properties of the carboxyl group
The Carboxyl Group: The Acidic Gatekeeper of Amino Acids
Imagine you’re traveling through a bustling city, dodging people and vehicles. Amidst the chaos, there’s a tiny building that stands out like a beacon of orderliness. This building is the carboxyl group, the gatekeeper of the amino acid world.
Like a doorman, the carboxyl group controls the flow of hydrogen ions. It’s a strong acid, meaning it readily donates these ions to create H+ ions that give it its acidic nature. But don’t be intimidated; the carboxyl group also has a softer side.
When the conditions are right, it undergoes a transformation, shedding its acidic nature and adopting a more basic personality. In this state, it becomes a carboxylate ion, receiving an extra negative charge. This switch in character is crucial for the carboxyl group’s interactions with other molecules.
So, if you’re ever lost in the labyrinth of amino acids, remember the carboxyl group as the regulatory beacon. It controls the acidity, participates in chemical reactions, and plays a critical role in the overall structure and function of proteins.
The Carboxyl Group: A Window into Acidic Properties
In the realm of amino acids, the carboxyl group takes center stage as the acidic counterpart. With its distinctive structure, it features an oxygen atom double-bonded to a carbon atom and a lone hydroxyl group (OH). This unique arrangement grants the carboxyl group its acidic nature.
When the carboxyl group interacts with water, a proton (H+) is released, giving rise to the formation of a carboxylate ion (COO-). This ionization process imbues the carboxyl group with its characteristic acidic properties. The ability to donate protons makes it a potent player in various chemical reactions and biological processes.
In the context of proteins, the carboxyl group’s acidity plays a crucial role in the formation of peptide bonds. These bonds, formed between two amino acids, are the backbone of protein structures. By donating a proton during bond formation, the carboxyl group establishes the amide linkage that holds the protein together.
Unlocking the Secrets of Amino Acids: Exploring the Amino Group
In the intricate world of biochemistry, amino acids stand as the fundamental building blocks of life’s most versatile molecules—proteins. Among their defining characteristics are the functional groups they possess, including the enigmatic amino group.
Structure and Chemical Properties of the Amino Group
Imagine a molecular structure adorned with a central nitrogen atom adorned with two shimmering hydrogen atoms. This is the essence of the amino group, a configuration denoted by the simple yet profound symbol -NH2. Its chemical nature bestows upon it the ability to behave as a base, a molecule that readily accepts protons.
Basicity and Protonation
When the amino group encounters an acidic environment, it undergoes a remarkable transformation. It attracts a wandering proton, forming a positively charged ammonium ion (-NH3+). This protonation reaction is a crucial factor in maintaining the equilibrium of amino acids and their role in biological processes.
Interplay with Other Functional Groups
Within the realm of amino acids, the amino group engages in a delicate dance with its counterparts, the carboxyl and side chain groups. Together, they orchestratea symphony of interactions that shape the molecule’s properties and pave the way for myriad functions.
In conclusion, the amino group is not merely a structural feature of amino acids; it is a dynamic force that governs their chemical behavior, paves the way for protein assembly, and ultimately orchestrates the orchestration of life’s essential processes.
The Amino Group: A Basic Building Block
In the realm of chemistry, there’s a fundamental unit that forms the backbone of proteins: the amino acid. Imagine a colorful Lego block, where each block represents a unique amino acid. Each block has a distinctive structure and properties, thanks to a trio of functional groups that adorn it.
One of these groups, the amino group, is a true star when it comes to alkalinity. It’s a chemical wizard that can make its surroundings more basic. Its structure resembles an umbrella, with a positively charged nitrogen atom at the tip, eager to share its positive vibes.
When the amino acid finds itself in a watery environment, its amino group unleashes its power. It grabs a proton, the tiny positive particle that makes acids acidic. This proton-grabbing ability transforms the amino group into a positively charged ion, known as the ammonium ion. In this new form, the amino acid becomes a veritable base, ready to neutralize acids and counter their sourness.
The transformation from amino group to ammonium ion is not just a passive bystander in the chemical drama of life. It’s an active participant that plays a pivotal role in shaping the properties and functions of amino acids and the proteins they form.
The Side Chain (R Group)
Introducing the side chain! This fascinating part of an amino acid is what gives it its unique personality. Just like a fashion accessory, the side chain can completely change the character of an amino acid. It can be polar or nonpolar, hydrophilic or hydrophobic. These properties determine how the amino acid interacts with its surroundings, much like how different accessories can influence our social interactions.
The side chain is often compared to a tree branch, stemming from the central carbon atom. It can be long or short, straight or branched, positively or negatively charged. This structural diversity contributes to the enormous variety of amino acids that can exist, each with its own unique function.
The side chain is like the special ingredient that gives each amino acid its distinctive flavor. It can be a chemical tool for interacting with other molecules or a building block for creating complex structures. Without the side chain, amino acids would be like plain vanilla ice cream – all the same and missing that extra something that makes them so versatile and essential.
A Tale of Amino Acids: The Side Chain’s Impact on Identity
In the realm of biomolecules, amino acids play a pivotal role as the building blocks of proteins. Each amino acid possesses a unique trio of functional groups: the carboxyl, amino, and side chain groups. While the carboxyl and amino groups are essential for linking amino acids together in protein chains, the side chain (R group) is like the fingerprint that distinguishes each amino acid, bestowing upon it distinct characteristics.
The side chain can vary greatly in its composition and properties. It can be polar, containing charged or uncharged groups that interact readily with water. Conversely, it can be nonpolar, lacking charged groups and preferring to reside in a hydrophobic environment. This diversity in side chains gives rise to the wide array of amino acids found in nature, each with its own unique role to play in the symphony of life.
Polar side chains love water’s embrace. They can form hydrogen bonds with water molecules, readily dissolving in aqueous solutions. Examples include aspartic acid, with its negatively charged carboxyl group, and lysine, boasting a positively charged amino group. These polar side chains facilitate interactions with other polar molecules, including proteins, nucleic acids, and carbohydrates. They also contribute to the hydrophilic nature of proteins, allowing them to dissolve in water and perform their functions in the aqueous environment of cells.
In contrast, nonpolar side chains shy away from water. They lack charged groups and prefer to interact with other nonpolar molecules, such as lipids and hydrocarbons. Examples include alanine, with its simple methyl group, and phenylalanine, with its bulky aromatic ring. These nonpolar side chains promote protein solubility in nonpolar environments, such as cell membranes. They also contribute to the hydrophobic interactions that stabilize protein structures, allowing proteins to maintain their intricate three-dimensional conformations.
The diversity of side chains among amino acids enables them to interact with a wide range of molecules. These interactions determine the chemical properties and biological functions of proteins. Polar side chains participate in hydrogen bonding and electrostatic interactions, facilitating protein solubility and protein-protein interactions. Nonpolar side chains promote protein-lipid interactions and contribute to protein stability. Together, these functional groups orchestrate the intricate dance of proteins, allowing them to perform their vital roles in cells and organisms.
The Significance of Side Chains in Amino Acids
Every amino acid possesses a distinguishing feature known as the side chain, or R group, which is a unique chemical structure attached to the central carbon atom. This unique appendage plays a crucial role in determining the properties and functions of each amino acid.
The side chain’s composition and characteristics influence polarity and hydrophobicity. Polar side chains, such as those containing hydroxyl or amino groups, have an affinity for water and are found in soluble proteins. In contrast, hydrophobic side chains, typically comprised of aliphatic or aromatic groups, repel water and are found in membrane-bound proteins.
The nature of the side chain also affects an amino acid’s interactions with other molecules. Negatively charged side chains, found in acidic amino acids like aspartic acid and glutamic acid, interact with positively charged side chains, such as those in basic amino acids like lysine and arginine, to form ionic bonds. These interactions play a key role in protein stability and function.
Furthermore, the side chains of amino acids can participate in specific chemical reactions, including hydrogen bonding, disulfide bond formation, and enzymatic catalysis. For instance, the side chain of cysteine contains a thiol group that can form disulfide bonds with other cysteine residues, contributing to protein structure and stability.
In summary, the side chain of an amino acid is a defining characteristic that significantly influences its properties and functions. It governs polarity, hydrophobicity, reactivity, and ultimately shapes the roles played by amino acids in the diverse biological processes that sustain life.
The Essential Roles of Functional Groups in Amino Acids
In the realm of proteins, amino acids stand as the fundamental building blocks. These biomolecules are adorned with three distinct yet interconnected functional groups: the carboxyl group, the amino group, and the side chain. Each group plays a crucial role in determining the chemical properties and biological functions of amino acids.
The carboxyl group (-COOH) embodies the acidic nature of amino acids. Its chemical composition endows it with the ability to donate protons (H+) and form carboxylate ions (-COO-). This characteristic imparts acidic properties to amino acids and plays a critical role in regulating pH levels within cells.
Conversely, the amino group (-NH2) serves as a basic group, capable of accepting protons and forming ammonium ions (-NH3+). By neutralizing acids, the amino group contributes to the alkaline nature of certain amino acids. Moreover, it participates in vital biochemical reactions, such as peptide bond formation.
The side chain (R group) emerges as a distinguishing feature among amino acids. Its diverse structural properties give rise to a broad range of chemical and biological characteristics. Some side chains are polar or hydrophilic, while others are nonpolar or hydrophobic. This diversity enables amino acids to interact with water or other molecules, ultimately shaping their roles in protein structure and function.
The collective interplay of these functional groups is what bestows unique properties upon each amino acid. They determine the molecule’s charge, solubility, and reactivity. Additionally, they facilitate molecular interactions that define the intricate structures and functions of proteins. Without the carboxyl, amino, and side chain groups, the world of proteins would be a mere skeleton of its current complexity.
In conclusion, the functional groups in amino acids are not mere appendages but rather essential actors in the molecular drama of life. Their presence orchestrates a symphony of chemical properties and biological functions, paving the way for the extraordinary diversity and versatility observed in the protein world.
Amino Acids: The Building Blocks of Life
In the realm of biomolecules, amino acids reign supreme, serving as the fundamental units that construct the intricate tapestry of proteins. These tiny but mighty molecules, each possessing unique characteristics, play a pivotal role in not only the structure but also the function of proteins.
At the heart of an amino acid lies a carboxyl group (-COOH), a functional group that bestows an acidic nature upon the molecule. This carboxyl group readily dissociates, releasing a hydrogen ion (H+) and forming a negatively charged carboxylate ion (-COO-).
On the opposite end resides an amino group (-NH2), an equally potent functional group that imparts a basic character. The amino group, conversely, can accept a hydrogen ion (H+), transforming into a positively charged ammonium ion (-NH3+).
But what truly sets amino acids apart is their side chain (R group), a distinctive appendage that varies from one amino acid to another. This side chain can be polar, nonpolar, or charged, influencing the molecule’s solubility and behavior in an aqueous environment.
The interplay between these functional groups orchestrates the biochemical dance of amino acids. The carboxylate and ammonium ions, with their opposing charges, can form ionic bonds, stabilizing the structure of proteins. Polar side chains, like -OH and -NH2, engage in hydrogen bonding, further strengthening protein architecture. Nonpolar side chains, such as hydrocarbons, prefer to associate with each other, creating hydrophobic interactions that contribute to protein folding and stability.
Beyond their structural roles, amino acids also actively participate in protein function. The R group’s unique properties can endow proteins with enzymatic activity, recognition capabilities, and regulatory functions. For instance, amino acids with charged side chains enable proteins to interact with ions and other charged molecules, while amino acids with hydrophobic side chains facilitate protein interactions with lipids and membranes.
In summary, the carboxyl, amino, and side chain functional groups of amino acids serve as the architects of protein structure and function. Their interactions and unique properties orchestrate a symphony of molecular interactions, enabling proteins to perform a vast repertoire of biological roles.