Amino Acids: Building Blocks Of Proteins With Versatile Functionality In Biological Processes
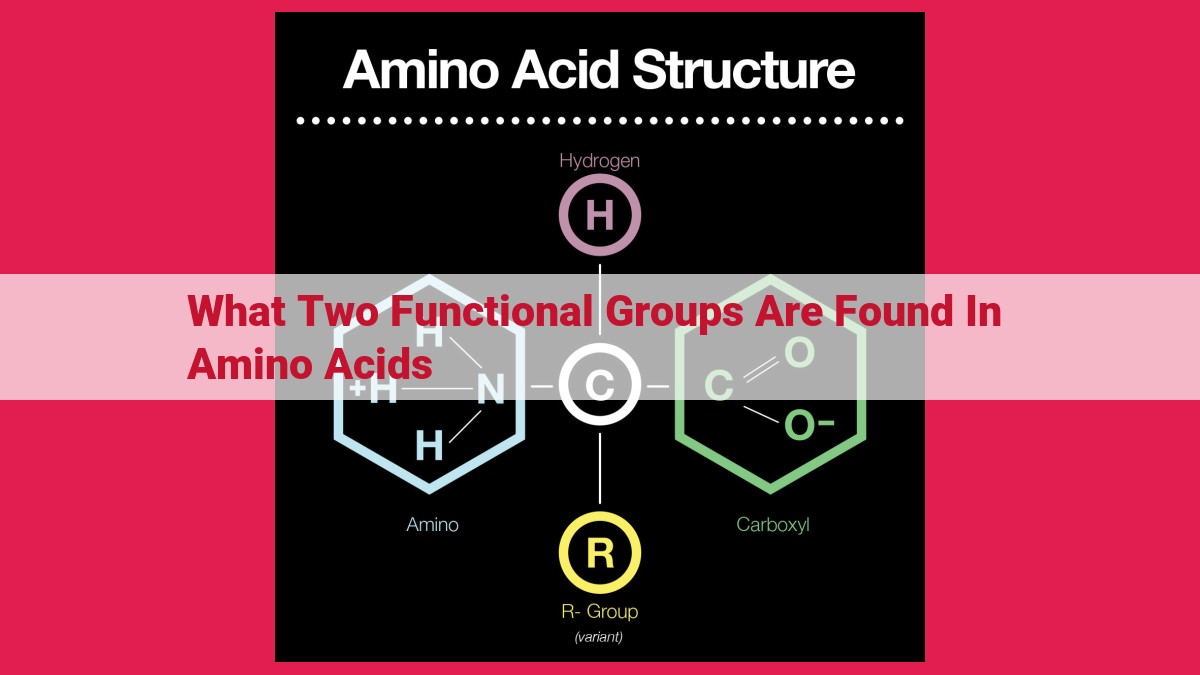
Amino acids, the building blocks of proteins, possess two key functional groups: the amino group (-NH2) and the carboxyl group (-COOH). The amino group, with its lone pair of electrons, allows amino acids to act as bases and participate in condensation reactions. The carboxyl group, with its acidic proton, enables amino acids to act as acids and form amides and esters. Together, these functional groups drive the formation of peptide bonds, linking amino acids into polypeptide chains, and endow amino acids with their versatile chemical reactivity crucial for biological processes.
Amino Acids: Unveiling the Building Blocks of Life
The Story of Life’s Foundation
In the intricate tapestry of life, a fundamental thread emerges—amino acids. These unassuming yet essential molecules serve as the building blocks of the proteins that shape our very existence. Like minuscule architects, they assemble and orchestrate the countless processes that sustain our biological machinery.
Understanding Amino Acids
Simply put, amino acids are organic compounds characterized by two defining functional groups: the amino group (-NH2) and the carboxyl group (-COOH). The amino group, with its lone pair of electrons, grants amino acids a basic character. Conversely, the carboxyl group, possessing a hydrogen atom bound to an oxygen atom double-bonded to a carbon atom, imparts an acidic nature.
These functional groups endow amino acids with remarkable versatility, enabling them to interact with a wide range of molecules through various chemical reactions. The dance between the amino and carboxyl groups, known as a condensation reaction, leads to the formation of peptide bonds—the backbone of proteins. These bonds thread amino acids together, creating polypeptide chains with diverse functions and intricate three-dimensional structures.
The Multifaceted Role of Functional Groups
The presence of these functional groups not only facilitates peptide bond formation but also empowers amino acids to participate in a vast array of other chemical reactions. This versatility is crucial for the myriad of biological processes that occur within living organisms.
For instance, the amino group enables amino acids to undergo amination reactions, where they donate amino groups to other molecules. The carboxyl group, on the other hand, allows for esterification reactions, forming esters that play vital roles in lipid metabolism.
Embracing Amino Acid Versatility
The uniqueness of amino acids lies in their ability to combine with other amino acids in innumerable ways. This diversity allows for the creation of a staggering array of proteins, each with its specific structure and function. Proteins orchestrate a symphony of cellular processes, from transporting molecules to catalyzing biochemical reactions, and they are indispensable for the proper functioning of living organisms.
In conclusion, amino acids, with their remarkable functional groups, are the cornerstones of life’s intricate architecture. They serve as the foundational units of proteins, which orchestrate the countless functions that sustain and define living beings.
Functional Group #1: The Amino Group (-NH2)
- Explain the structure and definition of the amino group.
- Describe its bonding capabilities and related concepts (amines, amides, ammonia, nitriles, nitro).
Functional Group #1: The Amino Group (-NH2)
In the realm of chemistry, amino acids stand as the essential building blocks of proteins, the very molecules that orchestrate life’s countless biological processes. Each amino acid boasts a unique identity, shaped by a central carbon atom adorned with a medley of functional groups. One such functional group, the amino group (-NH2), plays a pivotal role in the versatility and reactivity of amino acids.
Delving into the anatomy of the amino group, we encounter a solitary nitrogen atom bonded to two hydrogen atoms. This simple structure, pourtant, conceals a remarkable range of bonding capabilities. The nitrogen atom, eager for partners, readily forms covalent bonds with hydrogen atoms, giving rise to compounds known as amines. These amines, in turn, engage in further bonding adventures, creating amides and nitriles.
Venturing beyond the realm of amines, the amino group also forges alliances with other functional groups. When paired with a hydrogen atom, it transforms into ammonia, a colorless gas with a pungent odor. Conversely, when it encounters an oxygen atom bonded to a carbon atom, it forms a nitro group, notorious for its explosive properties.
The amino group‘s versatility extends far beyond its ability to form covalent bonds. It also harbors a secret weapon: its lone pair of electrons. This unattached pair of electrons eagerly participates in hydrogen bonding, a non-covalent interaction that fosters alliances between molecules.
In the context of amino acids, the amino group serves as a key player in the formation of peptide bonds, the covalent linkages that unite amino acids into protein chains. These peptide bonds, forged between the amino group of one amino acid and the carboxyl group of another, are the structural backbones of proteins, shaping their intricate folds and enabling their diverse functions.
In summary, the amino group (-NH2) of amino acids is a functional group of immense significance, bestowing upon these molecules a remarkable range of bonding capabilities and chemical reactions. Whether forming covalent bonds with hydrogen atoms, nitrogen atoms, or oxygen atoms, or participating in non-covalent hydrogen bonding, the amino group is the driving force behind the versatility and reactivity of amino acids, enabling them to play their crucial role as the building blocks of life.
Functional Group #2: The Carboxyl Group (-COOH)
At the heart of the amino acid’s functionality lies a versatile chemical group: the carboxyl group. Residing at the opposite end of the amino group, this enigmatic entity holds the key to unraveling the intricate web of amino acid interactions.
Structure and Definition of the Carboxyl Group
Imagine a carbon atom like a central stage, with two oxygen atoms hovering around it. One oxygen forms a double bond with the carbon, creating the carbonyl group. The other oxygen, armed with a lone pair of electrons, peeks out as a hydroxyl group, yearning for connection. This unique arrangement forms the carboxyl group, a chemical chameleon with remarkable bonding capabilities.
Bonding Capabilities and Related Concepts
The carboxyl group’s versatility stems from its ability to form a multitude of bonds. It can donate its hydroxyl hydrogen, becoming an acid. When paired with an alcohol, it transforms into an ester, linking two molecules together. The carboxyl group’s fondness for nitrogen extends to forming amides, essential for protein synthesis. It can also react with itself to create anhydrides, storing chemical energy for future reactions. Finally, the carboxyl group can accept protons to form salts, ensuring a balanced electrical landscape within cells.
The Role of Functional Groups in Amino Acid Reactions: A Tale of Peptide Bond Formation
In the realm of biochemistry, amino acids stand as the fundamental building blocks of life, each adorned with two indispensable functional groups: the amino group and the carboxyl group. These functional groups, like master puppeteers, orchestrate a myriad of chemical reactions that shape the destiny of amino acids and the proteins they assemble.
The Dance of Condensation Reactions
Condensation reactions mark a pivotal moment in the life of amino acids. In this intricate dance, the amino group of one amino acid meets the carboxyl group of another, entwining their fates like lovers in a tender embrace. As they fuse, a molecule of water is released as a testament to their union, and a new chemical bond emerges – the peptide bond.
The Genesis of Proteins: A Symphony of Peptide Bonds
Protein synthesis, the symphony of life, is orchestrated by a series of condensation reactions. Each peptide bond forged brings together two amino acids, linking them in a chain that can grow to astonishing lengths. These chains of amino acids, known as polypeptides, fold and weave themselves into intricate shapes, forming the proteins essential for life’s myriad functions.
The Versatility of Amino Acid Reactions
Beyond their role in peptide bond formation, amino acids are masters of disguise, capable of undergoing a kaleidoscope of other reactions thanks to their versatile functional groups. These reactions lie at the heart of countless biochemical processes, from cellular signaling to enzyme catalysis.
The amino group, with its nucleophilic nature, can participate in a plethora of reactions, including:
- Alkylation: Adding an alkyl group
- Acylation: Reacting with acyl chlorides or anhydrides
- Amide formation: Condensing with carboxyl groups
The carboxyl group, on the other hand, is an electrophile that readily undergoes reactions such as:
- Esterification: Reacting with alcohols to form esters
- Amide formation: Reacting with amino groups to form amides
- Decarboxylation: Removing the carboxyl group as carbon dioxide
The functional groups of amino acids play a pivotal role in the reactions that shape their destiny and the proteins they form. From the formation of peptide bonds to the versatility of various reactions, these functional groups are the unsung heroes of biochemistry, enabling the intricate dance of life.
Amino Acids: The Versatile Building Blocks of Life
At the heart of all biological processes lies the fundamental building block known as amino acids. These tiny molecules, crafted with precise functional groups, orchestrate a captivating dance of chemical reactions that sustains life as we know it.
Within the amino acid’s structure, two functional groups reign supreme: the amino group and the carboxyl group. Like a harmonized symphony, these groups engage in a delicate interplay, enabling amino acids to undergo a vast array of chemical reactions.
The amino group, with its reactive nitrogen atom, eagerly forms amides, essential for the very framework of proteins. These crucial bonds, forged through a condensation reaction, unite amino acids into intricate polypeptide chains, the foundation of life’s macromolecules.
Not to be outdone, the carboxyl group, with its acidic hydrogen, readily partakes in esterification and amide formation, further expanding the repertoire of amino acid reactions. These versatile groups, like skilled performers, don different roles in various biological processes.
From the synthesis of proteins to the regulation of enzymatic reactions, the functional groups of amino acids stand as indispensable players on the stage of life. Their innate reactivity enables the formation of countless molecules, each carrying out specific functions within the intricate tapestry of biological systems.
In essence, amino acids are not mere building blocks but rather versatile catalysts, orchestrating an ensemble of chemical reactions that drive the very essence of life. Their functional groups, like tiny choreographers, guide the dance that sustains the vibrant symphony we call biology.