Understanding Aluminum’s Valence Electrons: A Key To Its Chemical Behavior
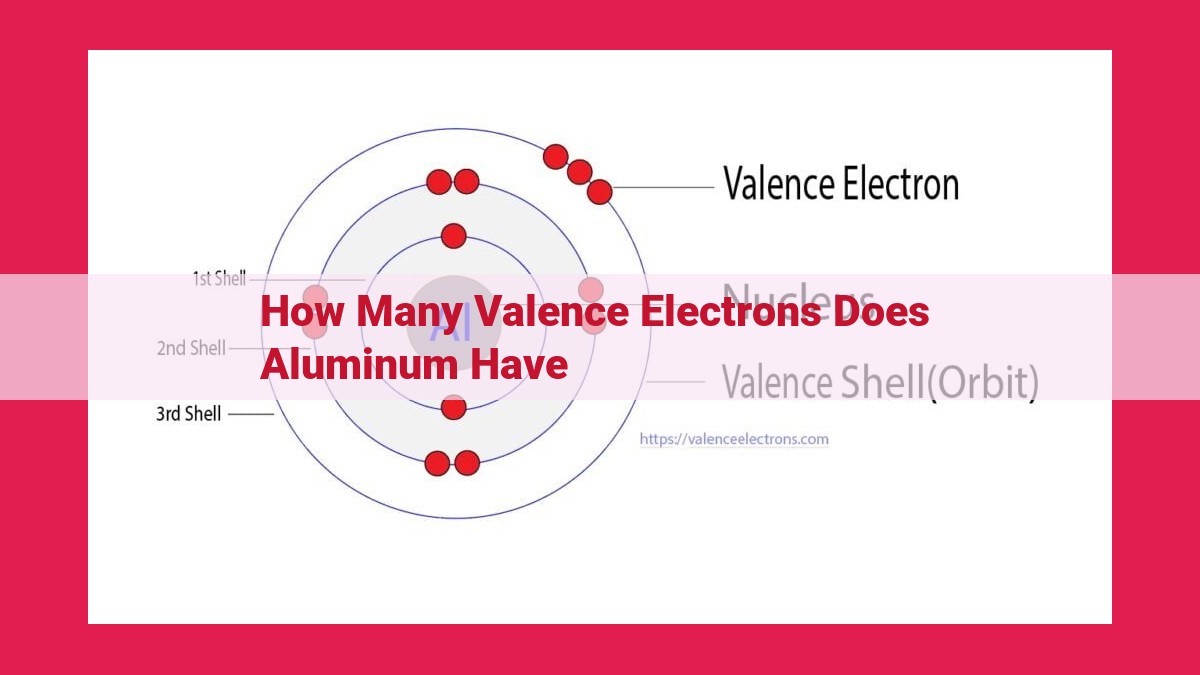
Aluminum has three valence electrons. These are the electrons in the outermost energy level of the atom, and they determine the chemical bonding behavior of the element. Aluminum is a metal in Group 13 of the periodic table, with atomic number 13. Its electron configuration is 2-8-3, with the three valence electrons in the 3p orbital. Valence electrons are crucial for chemical bonding, as they are the electrons that participate in the formation of covalent bonds with other atoms.
What Are Valence Electrons?
- Definition and role in chemical bonding and reactivity
- Explain the concept of electron configuration
What Are Valence Electrons? The Key to Chemical Bonding
In the realm of chemistry, understanding valence electrons is fundamental. These electrons, like tiny dancers, reside in the outermost energy level of an atom, eagerly participating in the captivating waltz of chemical bonding. They determine how atoms interact with each other, shaping the building blocks of our world.
Electron Configuration: Unveiling the Atomic Puzzle
Every atom is an intricate tapestry of electrons orbiting its nucleus. The electron configuration describes the arrangement of these electrons within the atom’s energy levels or orbitals. Think of it as a celestial map, guiding us through the ethereal landscape of the atom.
The valence electrons, the outermost inhabitants of this atomic realm, play a pivotal role in determining the atom’s chemical behavior. They are the social butterflies of the atom, eagerly engaging in chemical reactions to form bonds with other atoms.
In the periodic table, we find aluminum, a lightweight metal with a unique personality. Its atomic number, 13, signifies the precise number of protons in its nucleus, which is balanced by the same number of electrons.
Valence Electrons: A Crucial Factor in Aluminum’s Significance
Embarking on a journey into the realm of chemistry, we encounter the fascinating world of elements and their unique properties. Among these elements, aluminum stands out due to its ubiquity and the key role it plays in various industries. To unravel the secrets behind aluminum’s importance, we must delve into the concept of valence electrons, the cornerstone of chemical bonding and reactivity.
Aluminum: A Versatile Metal
Aluminum, the third most abundant element on Earth’s crust, belongs to Group 13 of the periodic table, sharing a special bond with other metals like gallium and indium. With an atomic number of 13, aluminum boasts 13 protons and electrons, giving it a distinctive atomic structure.
As a metal, aluminum exhibits remarkable characteristics that make it indispensable in a wide range of applications. Its strength, lightness, and corrosion resistance make it ideal for everything from aircraft construction to kitchen appliances. Aluminum’s malleability and ductility further enhance its usefulness, allowing it to be easily shaped and molded into various forms.
Electron Configuration of Aluminum: Unveiling the Secret of Chemical Bonding
What’s the Buzz About Valence Electrons?
Valence electrons are like the social butterflies of the atomic world, determining an element’s bonding potential and reactivity. For aluminum, an intriguing element in Group 13, these electrons hold the key to its chemical adventures.
Meet the Aluminum Atom: A Metal with a Twist
In the grand cosmic tapestry of elements, aluminum stands out as a silvery-white metal with a remarkable ability to shape the world around us. Its atomic number of 13 reveals a nucleus surrounded by 13 electrons. But it’s the arrangement of these electrons that truly sets aluminum apart.
Delving into the Electron Configuration: A Tale of Orbitals and Energy Levels
Picture the aluminum atom as a miniature solar system. Electrons, like tiny planets, orbit the central nucleus in specific energy levels or shells. The innermost shell, closest to the nucleus, is the ground floor of the atomic mansion, followed by higher floors or energy levels. Each shell can accommodate a certain number of electrons, like a celestial apartment building.
In aluminum’s case, the first energy level is a cozy one-bedroom apartment, housing two electrons. The second energy level expands to four bedrooms, sheltering eight electrons. But it’s the third and outermost energy level that holds the key to aluminum’s chemistry. Here, we find three valence electrons, like mischievous kids playing in the backyard, eagerly awaiting the opportunity to join the bonding dance.
The Significance of Valence Electrons in Aluminum
Understanding Valence Electrons
In the realm of chemistry, valence electrons play a pivotal role in shaping the behavior and reactivity of elements. These electrons reside in the outermost energy level of an atom and determine its ability to form chemical bonds.
Introducing Aluminum
Aluminum, a member of Group 13, is a lightweight and versatile metal with a unique electron configuration. Its atomic number, 13, indicates the presence of 13 electrons, including 3 valence electrons in its outermost energy level.
Valence Electrons and Chemical Bonding
Valence electrons dictate how an element interacts with others. In the case of aluminum, its three valence electrons seek to achieve a stable configuration by either gaining or losing electrons. This drive for stability determines the types of chemical bonds that aluminum forms.
Implications for Aluminum’s Bonding Behavior
The presence of three valence electrons in aluminum suggests that it can:
- Form ionic bonds: Aluminum can lose its valence electrons to achieve a stable octet configuration, creating positively charged ions. These ions can then form ionic bonds with negatively charged ions, such as chlorine.
- Form covalent bonds: Aluminum can also share its valence electrons with other atoms, forming covalent bonds. This allows aluminum to form compounds with non-metals, such as oxygen in aluminum oxide.
By understanding the role of valence electrons, we Gain insights into the chemical versatility of aluminum. Its ability to participate in both ionic and covalent bonding makes it a valuable component in a wide range of industrial and consumer products.