Aluminum’s Electron Configuration: Unlocking Chemical Properties And Industrial Significance
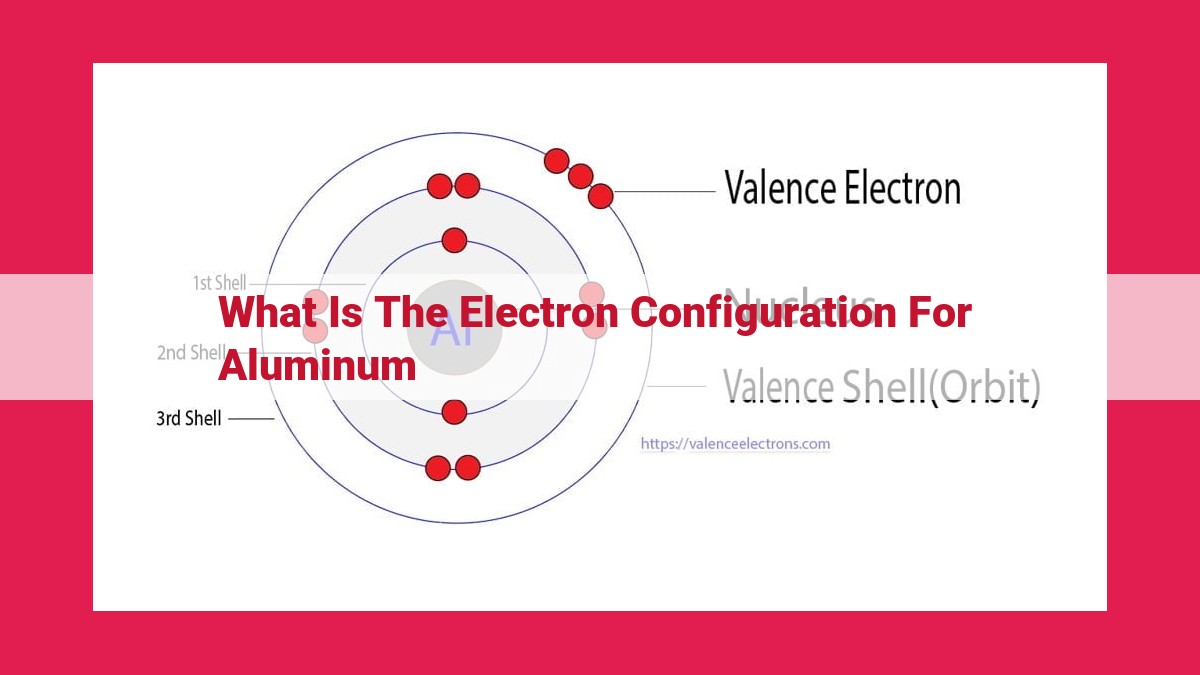
Aluminum’s electron configuration, 1s22s22p63s23p1, describes the arrangement of its 13 electrons within its atomic orbitals. It signifies aluminum’s atomic number, determining its chemical properties and reactivity. The presence of one valence electron makes aluminum a reactive metal and an essential component in various alloys and compounds.
Understanding Electron Configuration: A Journey into the Atomic Realm
In the world of chemistry, unraveling the secrets of the atom is a fundamental pursuit. Electron configuration, a cornerstone of atomic structure, unlocks a deeper comprehension of chemical behavior. Let’s embark on a thrilling expedition into this intriguing domain.
Electron configuration, in essence, portrays the arrangement of electrons within an atom’s energy levels. These electrons, the subatomic particles with a negative charge, reside in specific shells or energy levels around the atom’s nucleus. The number of shells and the way electrons fill them govern an atom’s chemical properties.
For instance, let’s consider aluminum. With an atomic number of 13, aluminum possesses 13 electrons. Of these, three electrons occupy the outermost shell, making them valence electrons. These valence electrons play a crucial role in determining aluminum’s chemical reactivity and bonding behavior.
Exploring Related Concepts in Electron Configuration
To delve deeper into the realm of electron configuration, let’s explore some fundamental concepts that intertwine with its understanding.
Protons, Mass Number, and Isotopes
Every atom is comprised of a central nucleus containing positively charged protons and neutral neutrons. The mass number represents the total number of protons and neutrons present in an atom’s nucleus. Atoms of the same element can have varying numbers of neutrons, known as isotopes. Isotopes possess different mass numbers despite sharing an identical atomic number.
Chemical Bonding and Electron Dot Diagrams
Electron configuration plays a pivotal role in determining an atom’s chemical behavior. Atoms strive to achieve a stable electron configuration resembling the inert gases. Bonding occurs when atoms interact to fulfill their electron requirements. Electron dot diagrams depict the arrangement of valence electrons in an atom, which are involved in chemical reactions. By analyzing electron dot diagrams, we can predict the types of bonds formed between atoms.
Atomic Orbitals, Quantum Numbers, and Periodic Trends
The electron configuration of an atom is governed by the distribution of electrons in specific atomic orbitals. These orbitals have unique quantum numbers that describe the energy, shape, and orientation of the electron within the orbital. The periodic trends observed in the periodic table reflect the similarities in electron configurations and chemical properties of elements within the same group or period.
Uncovering Electron Stability: The Alchemy of Atoms
Every atom, the fundamental building block of matter, possesses an intricate dance of electrons orbiting its nucleus. Electron stability, a crucial aspect of atomic chemistry, determines the behavior and reactivity of these subatomic particles.
In the world of electron stability, the inert gases reign supreme. These elements, such as helium, neon, and argon, have their outermost energy levels completely filled with electrons. This electronic contentment renders them chemically inert, as they have no driving force to gain or lose electrons.
Another significant concept in electron stability is ionic bonding. When an electropositive element (e.g., sodium) transfers one or more valence electrons to an electronegative element (e.g., chlorine), they form an ionic compound. The resulting ions, with their opposite charges, are held together by electrostatic attraction.
For instance, when sodium (Na) transfers an electron to chlorine (Cl), it becomes positively charged (Na+) while chlorine becomes negatively charged (Cl-). This transfer stabilizes both atoms, giving them inert gas-like electron configurations.
Understanding electron stability is paramount for comprehending the behavior of atoms and molecules. It forms the foundation for predicting chemical properties, explaining bonding patterns, and unraveling the fascinating tapestry of chemistry.
Molecular Orbitals and Bonding: The Dance of Electrons
In the realm of chemistry, the concept of electron configuration holds a pivotal role. It provides a roadmap to the arrangement of electrons within an atom, influencing its chemical behavior and bonding properties.
As we delve into the world of molecular orbitals, we uncover the fascinating dance of electrons that governs the formation of chemical bonds. These molecular orbitals are areas in space where electrons congregate and interact with each other.
Hybridization is a crucial process that reshapes atomic orbitals into new hybrid orbitals with distinct shapes and energies. This hybridization enables the formation of stronger and more stable bonds.
The concept of bond order quantifies the strength of a chemical bond. It represents the number of electron pairs shared between two atoms. Higher bond orders indicate stronger and more stable bonds.
Through the lens of molecular orbitals and bonding, we gain a profound understanding of the intricate ways in which atoms interact to form molecules. These concepts provide essential insights into the properties and behavior of various chemical compounds, shaping our understanding of the world around us.
Practical Applications of Electron Configuration
Understanding electron configuration not only provides insights into the fundamental nature of elements but also has far-reaching practical applications that directly impact our daily lives. One such application is the ability to predict chemical properties and reactivity.
Every element’s chemical behavior is largely determined by its electron configuration. For instance, aluminum, with its three valence electrons, exhibits a strong tendency to form chemical bonds to achieve a stable electron configuration. This property makes aluminum a highly reactive metal, allowing it to be used in a wide range of industrial and commercial applications, including construction, cookware, and beverage cans.
Similarly, electron configuration can explain bonding behavior in aluminum compounds. Aluminum’s three valence electrons make it a versatile bonding agent, capable of forming both covalent and ionic bonds. Covalent bonds, where electrons are shared between atoms, are responsible for the formation of aluminum compounds like aluminum chloride (AlCl3). In contrast, ionic bonds, where electrons are transferred between atoms, result in compounds like aluminum oxide (Al2O3). Understanding electron configuration allows chemists to tailor aluminum compounds with specific properties for various technological applications.
The Importance of Electron Configuration
Electron configuration, the arrangement of electrons in an atom’s energy levels, is a fundamental concept in chemistry. It plays a decisive role in determining an element’s properties, influencing its reactivity, bonding behavior, and overall chemical identity.
Electron configuration explains the stability of certain atomic arrangements. The noble gases, with their filled valence shells, are highly stable and chemically inert. This observation led to the concept of electron stability, which suggests that atoms tend to rearrange their electrons to achieve a noble gas configuration.
Understanding electron configuration enables us to predict chemical behavior and explain bonding in various compounds. For example, aluminum, with three valence electrons, forms ionic bonds by readily losing these electrons to acquire a stable octet configuration.
Moreover, electron configuration has numerous practical applications:
- In materials science, it guides the design of semiconductors and conductors by controlling the number and arrangement of valence electrons.
- In chemistry, it predicts the reactivity and stability of molecules, aiding in the development of new compounds and drugs.
- In astrophysics, it explains the spectral lines observed from stars, providing insights into the elemental composition and properties of celestial bodies.
Electron configuration serves as a cornerstone of chemical knowledge, guiding us in understanding the intricate world of atomic interactions and their practical implications. It empowers us to predict, manipulate, and harness the properties of matter, advancing scientific breakthroughs and shaping our technological landscape.