Alkali Metals: Unraveling The Chemistry Of Lone Valence Electrons
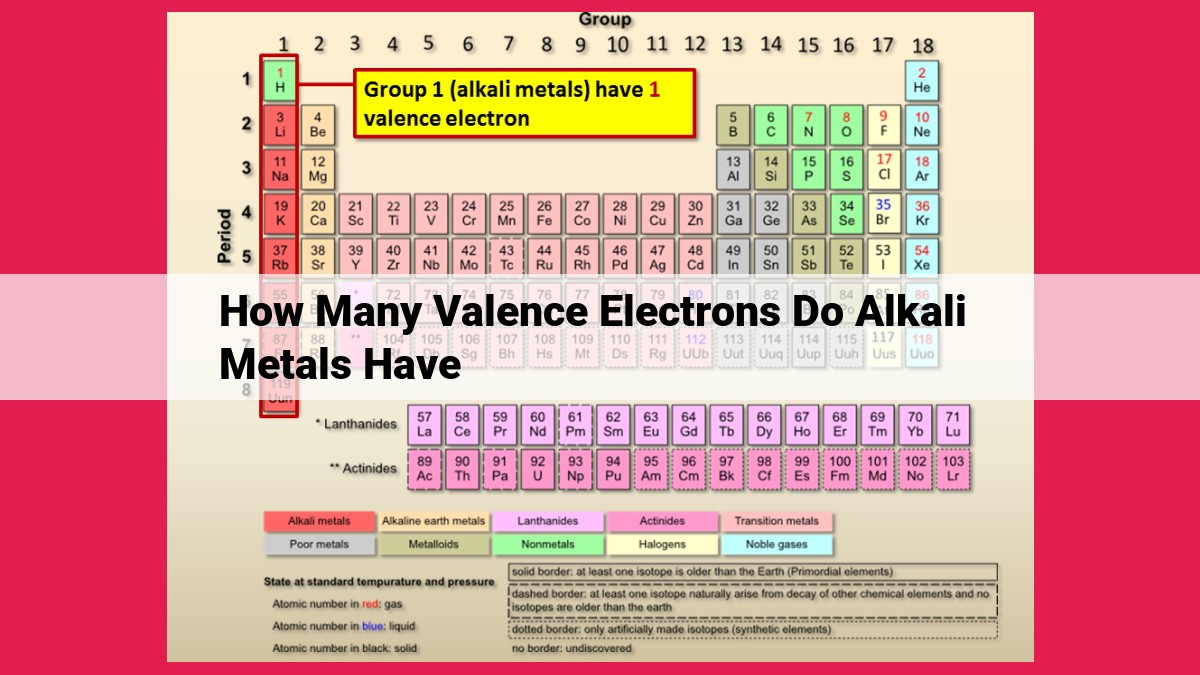
Alkali metals, known for their lone valence electron, possess a unique chemical identity. This single valence electron in their outermost energy level determines their reactivity and bonding behavior. Alkali metals readily shed this valence electron, forming ionic bonds with non-metals, resulting in their characteristically high reactivity. The presence of only one valence electron sets them apart in the periodic table, making them the quintessential example of elements with a singular valence electron configuration.
Valence Electrons: The Building Blocks of Chemical Bonding
- Definition and location of valence electrons
- Their crucial role in determining an atom’s chemical behavior
Valence Electrons: The Building Blocks of Chemical Bonding
In the vast tapestry of chemistry, the concept of valence electrons takes center stage. These are the electrons that reside in the outermost energy level of an atom, and they play a crucial role in shaping the atom’s chemical behavior.
Imagine valence electrons as the building blocks of chemical bonding. They determine how an atom interacts with other atoms, forming the strong bonds that create molecules. The number of valence electrons an atom possesses dictates its craving for chemical connections.
The location of valence electrons is essential in understanding their behavior. They reside in specific orbitals, which are distinct regions of space surrounding the atom’s nucleus. These orbitals can hold a maximum of two electrons, and they dictate the electron’s energy and shape.
Alkali Metals: The Lone Rangers of the Periodic Table
In the realm of chemistry, where elements dance and interact, the alkali metals stand out as lone rangers, possessing a unique characteristic that sets them apart from the rest of the periodic table: they have only one valence electron. This singular feature not only defines their chemical behavior but also makes them highly reactive.
The alkali metals, residing in the first column of the periodic table, consist of lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr). These elements share a common ancestry, inheriting the lone valence electron that resides in their outermost energy level.
The Lone Valence Electron: A Defining Trait
This single valence electron acts like an unstable wanderer, eager to escape the atom’s embrace. Its presence makes the alkali metals highly electropositive, meaning they have a strong tendency to donate their valence electron and become positively charged ions.
Why This Feature Sets Them Apart
The alkali metals’ unique electron configuration has profound implications for their chemical properties. Their low ionization energy (the ease with which they lose their valence electron) makes them highly reactive, especially with non-metals. This reactivity governs many of their defining characteristics.
For example, alkali metals readily form ionic bonds with non-metals, where they transfer their valence electron to the non-metal, creating a stable octet of electrons in both atoms. This explains their silvery-white appearance, soft and malleable nature, and low melting and boiling points.
In conclusion, the lone valence electron of the alkali metals is their defining trait, shaping their chemical behavior and reactivity. It sets them apart as lone rangers in the periodic table, influencing their interactions with other elements and giving rise to their unique properties.
Reactivity Unraveled: Valence Electrons Drive Chemical Bonds
In the bustling world of chemistry, valence electrons take center stage as the key players in chemical bonding. These electrons, residing in the outermost shell of atoms, are the architects of chemical reactions. When it comes to reactivity, alkali metals shine as the ultimate rule-breakers, thanks to their unique valence electron configuration.
Alkali metals, like mischievous pranksters, flaunt their single valence electron. Unlike other elements, they eagerly shed this lone electron, revealing their playful nature. This eagerness to part ways with their electron grants them an impressive reactivity. They just can’t resist the allure of a good chemical bonding adventure!
When alkali metals encounter non-metals, fireworks ensue. The non-metals, craving an extra electron to complete their valence shell, gladly accept the alkali metals’ offering. This electron transfer, like a handshake between atoms, forms an ionic bond. The alkali metal loses an electron, becoming a positively charged cation, while the non-metal gains an electron, transforming into a negatively charged anion.
These ionic bonds, akin to the unwavering bonds of friendship, hold the alkali metals and non-metals together, creating stable compounds. The high reactivity of alkali metals, fueled by their single valence electron, lies at the heart of their remarkable ability to form these ionic bonds. Their eagerness to shed their electron transforms them into bonding enthusiasts, playing a pivotal role in the intricate tapestry of chemical reactions.