Air Cooling Processes: Adiabatic, Evaporative, And Radiational Cooling
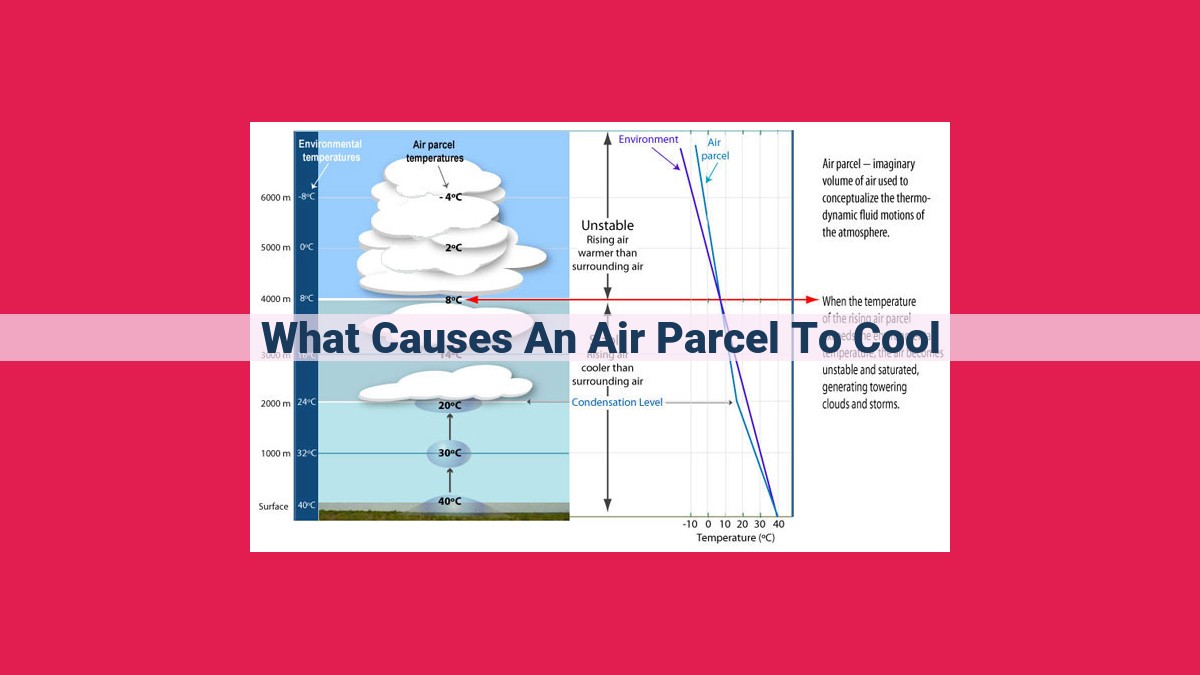
An air parcel cools when it experiences adiabatic cooling as it rises and expands, releasing heat due to decreased pressure. Evaporation also cools air by absorbing heat from its surroundings as water transforms into vapor. Additionally, radiational cooling occurs when objects emit infrared radiation, transferring heat to the surrounding air and causing it to cool.
Adiabatic Cooling: As Air Takes Flight
In the realm of Earth’s atmosphere, a fascinating phenomenon known as adiabatic cooling comes into play. It’s a cooling process that occurs as air parcels rise and expand, interacting with the surrounding environment.
As an air parcel ascends, it encounters lower atmospheric pressure. This causes the air molecules within the parcel to spread out, increasing their volume. During this expansion, no heat is exchanged with the surrounding air. Thus, the air parcel loses internal energy, resulting in a decrease in its temperature. This is the essence of adiabatic cooling.
The rate of adiabatic cooling is influenced by compressibility, which measures how easily the air parcel can be compressed. If the air is highly compressible, it will cool more rapidly as it rises. Conversely, if the air is less compressible, the cooling rate will be slower. This is because highly compressible air requires less energy to expand, while less compressible air requires more energy.
Evaporation: Water’s Cooling Magic
In the tapestry of nature’s cooling mechanisms, evaporation stands out as a gentle yet powerful force. It’s a process that transforms liquid water into an invisible vapor, simultaneously relieving the surrounding air of its oppressive heat.
How Evaporation Works
Imagine a tiny water molecule trapped on the surface of a liquid. It vibrates with energy, jostling against its neighbors. With enough energy, it breaks free from its watery confinement and escapes into the air as vapor. This escape is not without cost. The fleeing molecule carries away with it a substantial amount of heat, leaving the remaining water cooler.
Factors Influencing Evaporation
The rate at which evaporation occurs is influenced by several key factors:
- Vapor Pressure: This is the pressure exerted by water vapor in the air. The higher the vapor pressure, the slower the evaporation rate, as the air is already saturated with water.
- Boiling Point: The temperature at which water rapidly transforms into vapor. Lower boiling points lead to faster evaporation rates.
- Heat of Evaporation: The amount of heat required to turn one gram of liquid water into vapor. Substances with higher heat of evaporation evaporate more slowly.
The Cooling Effect
As water evaporates, it absorbs heat from its surroundings, creating a cooling effect. This is why sweating cools us down. When sweat evaporates from our skin, it draws heat away from our bodies, leaving us feeling refreshed.
The rate of evaporation is crucial in determining the effectiveness of cooling. In arid environments, where the air is dry and vapor pressure is low, evaporation occurs rapidly, leading to efficient cooling. Conversely, in humid environments, where the air is already saturated with water, evaporation slows down and cooling is less effective.
Evaporation plays a vital role in regulating the Earth’s climate. It transports heat from the oceans to the atmosphere, where it is released through clouds and precipitation. This process helps to maintain the Earth’s temperature balance and prevents the planet from overheating.
Radiational Cooling: The Night Sky’s Embrace
As the sun dips below the horizon and twilight’s embrace engulfs the world, a hidden force emerges from the depths of the cosmos, gently cradling the Earth in its ethereal grip. This enigmatic phenomenon, known as radiational cooling, orchestrates a symphony of nature’s finest instruments, weaving a tapestry of coolness that pervades the sleeping landscape.
In the realm of radiational cooling, all matter plays a part. Objects possess an inherent ability to emit infrared radiation, an invisible form of energy that carries heat away from their surfaces. The intensity of this radiation depends on the object’s emissivity, which gauges its proficiency in releasing infrared waves into the atmosphere.
Just as objects emit infrared radiation, they also absorb radiation from their surroundings. However, the degree to which they absorb radiation varies based on their absorptivity. Objects with high absorptivity readily soak up infrared energy, while those with low absorptivity act as reflective barriers, bouncing these waves away.
The dance between emission and absorption determines the fate of the Earth’s surface temperature after nightfall. As the sun retreats, objects on the ground emit infrared radiation unimpeded, sending their warmth toward the sky. However, the atmosphere, composed primarily of air parcels, acts as a selective filter. Air parcels readily absorb infrared radiation emitted by objects with high emissivity, effectively trapping heat near the Earth’s surface. In contrast, air parcels have minimal absorption capacity for infrared radiation emitted by objects with low emissivity, allowing the heat to escape freely into the vast expanse of space.
This disparity in heat trapping efficiency creates a gradient of cooling, with objects possessing high emissivity experiencing more pronounced cooling than those with low emissivity. Over time, the cumulative effect of radiational cooling yields a tangible difference in temperature, gradually transforming the world into a cooler and more serene sanctuary under the celestial canopy.