How Aerobic Respiration Generates Energy: Oxygen’s Vital Role
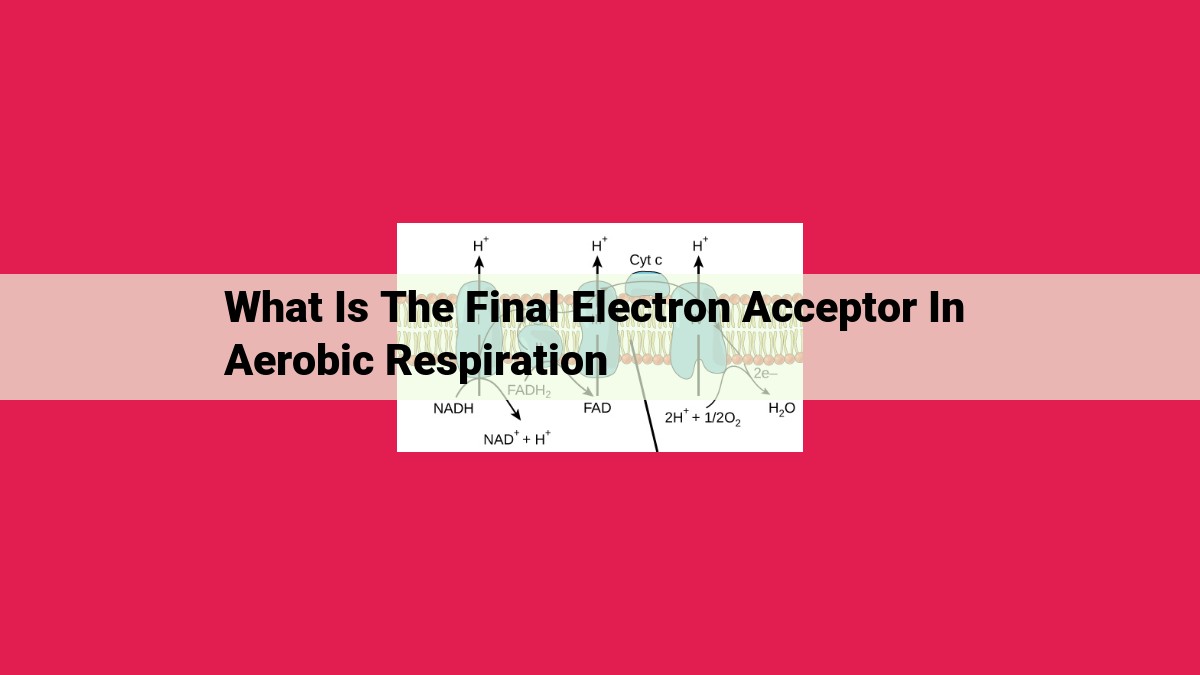
In aerobic respiration, the final electron acceptor is oxygen. Oxygen is the ultimate recipient of electrons passed through the electron transport chain, a series of protein complexes within mitochondria. This process, known as oxidative phosphorylation, generates most of the ATP (energy currency) required by cells. Oxygen’s absence during respiration leads to anaerobic respiration, which utilizes alternative electron acceptors and produces less ATP.
Unveiling the Final Electron Acceptor in Aerobic Respiration
In the intricate world of cellular energy production, understanding the final electron acceptor in aerobic respiration holds paramount importance. Aerobic respiration, a process in which glucose is broken down in the presence of oxygen, is the primary energy source for most living organisms. The electron acceptor in this process serves as a crucial determinant of the efficiency and sustainability of energy production.
Oxygen: The Ultimate Electron Sink
As we delve into the realm of aerobic respiration, we encounter oxygen as the key player, the final electron acceptor that ultimately receives electrons from the electron transport chain (ETC). This unique characteristic differentiates aerobic respiration from anaerobic respiration, where molecules other than oxygen, such as sulfate or nitrate, accept electrons.
The Electron Transport Chain: A Highway for Electrons
Electrons, the fundamental units of energy transfer, flow through a series of electron carriers within the ETC, a complex system located within mitochondria. Starting with NADH and FADH2, electrons are passed along a series of cytochromes, protein complexes that facilitate electron transfer. As electrons move through the ETC, their energy is harnessed to pump protons across the mitochondrial membrane.
Oxidative Phosphorylation: Harvesting Energy
The accumulation of protons outside the mitochondrial membrane creates a concentration gradient, a driving force that powers oxidative phosphorylation. ATP synthase, a molecular machine, utilizes this gradient to drive the synthesis of ATP (adenosine triphosphate), the universal energy currency of cells. ATP provides the energy required for cellular functions, fueling everything from muscle contraction to brain activity.
Importance of Understanding the Final Electron Acceptor
Unveiling the nature of the final electron acceptor in aerobic respiration is not merely an academic pursuit. This knowledge has profound implications in various fields:
- Medicine: Understanding the role of oxygen as the final electron acceptor is critical in treating conditions such as hypoxia (low oxygen levels) and hyperoxia (high oxygen levels).
- Biochemistry: The study of electron acceptors provides insights into the mechanisms of energy production, with applications in biotechnology and drug development.
- Environmental Science: Exploring the effects of changing oxygen levels on microbial processes in soil and water environments can help mitigate pollution and promote biodiversity.
Oxygen: The Ultimate Electron Acceptor in Aerobic Respiration
In the intricate dance of life, cells engage in a ceaseless process called respiration, the very heartbeat of their existence. Aerobic respiration stands out as the most efficient form, utilizing the presence of oxygen as its indispensable final electron acceptor.
Unlike anaerobic respiration, which operates in the absence of oxygen, aerobic respiration flourishes in its presence, unlocking a remarkable energy yield. Oxygen has a **singular” role to play as the final electron acceptor, guiding electrons through a series of redox reactions, ultimately leading to the generation of cellular energy in the form of Adenosine Triphosphate (ATP).
This fundamental distinction between aerobic and anaerobic respiration lies in their choice of electron acceptors. While aerobic respiration relies on oxygen, anaerobic respiration employs alternative electron acceptors, such as nitrate or sulfate. Aerobic respiration thus stands as the **more efficient and versatile” pathway, particularly in oxygen-rich environments like our own.
The Final Electron Acceptor: A Closer Look
In the intricate dance of aerobic respiration, the final electron acceptor plays a crucial role. Understanding its identity is essential to unraveling the secrets of this fundamental process.
What’s an Electron Acceptor?
Electron acceptors are molecules that eagerly receive electrons from electron donors. In aerobic respiration, the final electron acceptor is none other than oxygen (O2). Oxygen acts as the ultimate recipient of electrons from the electron transport chain, the power plant of the cell.
The Electron Transport Chain
The electron transport chain is a series of protein complexes embedded in the inner mitochondrial membrane. Electron carriers, such as NADH and FADH2, deposit their precious electrons onto the chain. These electrons embark on a thrilling journey, hopping from one complex to the next, losing energy as they travel.
Oxidative Phosphorylation: Energy Harvest
The crucial process of oxidative phosphorylation harnesses the released energy to synthesize ATP. ATP, the cellular energy currency, powers countless processes within the cell. The electron transport chain, fueled by the flow of electrons, creates a proton gradient across the inner mitochondrial membrane. This gradient drives the ATP synthase complex, much like a hydroelectric dam harnesses the power of flowing water.
Significance of Oxidative Phosphorylation
Without oxidative phosphorylation, the electron transport chain would be a futile exercise. It’s this process that captures the energy from electron flow and transforms it into the energy-rich ATP. ATP fuels a multitude of cellular activities, including muscle contraction, nerve impulse transmission, and chemical synthesis. It’s the lifeblood that drives the cell forward, making oxidative phosphorylation an indispensable step in aerobic respiration.
The Electron Transport Chain: Electron Highway
Within the mysterious depths of our mitochondria, there lies a hidden pathway—the electron transport chain—an essential component of aerobic respiration that powers our lives. Like a metaphorical highway, it carries electrons, the energetic messengers of life, along a carefully orchestrated journey.
The electron transport chain is a complex array of membrane-bound proteins nestled within the inner membrane of mitochondria. It’s here that the electrons, harvested from glucose and carried by NADH and FADH2, embark on their electronic odyssey.
NADH and FADH2, the primary electron carriers, deliver their precious cargo to the first protein complex in the chain. As electrons flow through this protein highway, they encounter a series of cytochromes, essential protein molecules that facilitate the transfer of electrons. Like tiny electron-carrying shuttles, cytochromes move electrons down a gradient of energy levels, releasing energy with each step.
This cascading flow of electrons powers a remarkable process known as oxidative phosphorylation, the key to our energy production. The electron transport chain pumps protons across the mitochondrial inner membrane, creating a proton gradient—a reservoir of potential energy. As protons rush back down this gradient, they pass through a protein complex called ATP synthase, which captures their energy and uses it to convert ADP into ATP, the universal energy currency of cells.
The electron transport chain, the electron highway within our mitochondria, is a constant hub of activity. It’s the site where electrons are transported, energy is harvested, and life’s essential energy currency is produced. Understanding the electron transport chain is essential for comprehending the very essence of cellular respiration—the process that sustains our vibrant lives.
Oxidative Phosphorylation: Harnessing Energy from Electron Flow
- Description of ATP synthase and its role in ATP synthesis
- Formation of a proton gradient as a driving force
- Correlation between electron transport chain activity and ATP production
Oxidative Phosphorylation: The Powerhouse of the Cell
The electron transport chain, the final stage of aerobic respiration, resembles a bustling highway where electrons dance to a symphony of energy production. As electrons journey through this molecular maze, their movement fuels an electrochemical gradient that powers the cell’s energy engine: oxidative phosphorylation.
At the heart of oxidative phosphorylation lies ATP synthase, a molecular machine that synthesizes ATP, the universal currency of energy in cells. This tiny dynamo harnesses the energy generated by the electron transport chain, creating a proton gradient across the mitochondrial membrane. The gradient drives protons back through channels in ATP synthase, causing it to rotate and pump out ATP.
The correlation between electron transport chain activity and ATP production is a testament to the efficiency of this biological process. As electrons flow through the chain, they generate a larger proton gradient, driving ATP synthase to produce more ATP. This intricate dance of electrons and protons ensures a steady supply of energy for the cell’s myriad activities.