Advanced Separation Techniques: Unraveling The Secrets Of Compound Analysis
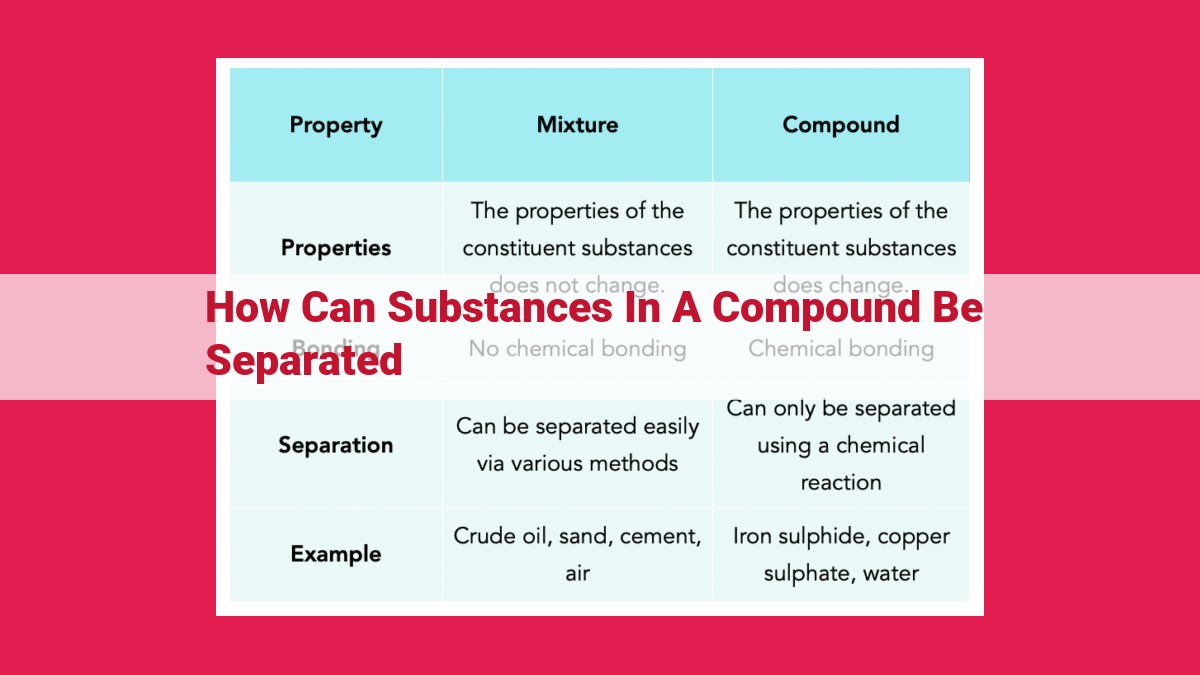
Separating substances in a compound is crucial for various scientific and industrial processes. Techniques like filtration, decantation, centrifugation, chromatography, distillation, sublimation, crystallization, extraction, and magnetic separation utilize different principles to separate substances based on size, density, affinity, boiling point, solubility, magnetic properties, and more. These techniques allow for the isolation, purification, and analysis of compounds, playing vital roles in fields such as chemistry, biology, pharmaceuticals, and engineering.
Substance Separation: The Art of Uniting and Purifying Matter
The world around us is a complex symphony of substances, each with its unique properties. Understanding how to separate these substances is a fundamental skill in various fields, from chemistry and biology to environmental science and manufacturing.
Why Substance Separation Matters
The ability to separate substances allows us to:
- Identify and analyze the components of complex mixtures
- Purify materials for specific applications
- Extract valuable substances from natural sources
The Secret to Separation: Exploiting Differences
The key to successful substance separation lies in exploiting the differences between the substances we wish to separate. These differences can be physical (such as size, density, or solubility) or chemical (such as affinity for certain solvents). By understanding these differences, we can select the appropriate separation technique to achieve our desired outcome.
Filtration: A Tale of Separation Based on Size
In the vast realm of science and industry, the ability to separate substances is paramount. Among the myriad of techniques used for this purpose, filtration stands out as a simple yet effective method for separating particles based on their size.
Imagine a filter paper, a thin, porous material that acts as a physical barrier. As a suspension of particles flows through the paper, larger particles become trapped in the pores, while smaller ones pass through. This process is akin to a delicate dance, where each particle is tested for its size and either retained or allowed to continue its journey.
The principle behind filtration lies in the size discrepancy between the particles and the pores of the filter paper. Larger particles, with their bulky dimensions, cannot squeeze through the narrow passages, while smaller ones, with their nimble forms, can navigate the labyrinth with ease.
The versatility of filtration extends to a wide range of applications. In laboratories, it is used to purify solutions by removing solid impurities. In industries, it is employed to separate dust particles from gases, ensuring cleaner air for workers and the environment. Even in our daily lives, filtration plays a vital role, as coffee filters prevent grounds from entering our morning brew.
So, the next time you witness a substance being separated through filtration, take a moment to appreciate the intricate interplay of size and porosity that underlies this fundamental technique. It is a testament to the power of human ingenuity and the beauty of scientific principles at work.
Decantation: Separating Liquids Based on Density
In the realm of substance separation, decantation emerges as a simple yet effective technique for parting liquids that differ in their density. This process involves carefully pouring the top layer of a liquid mixture into another container, leaving the denser liquid behind. It’s like gently separating oil from water – the oil, being lighter, floats on top and can be skimmed off.
Decantation finds its applications in various fields, and its simplicity makes it accessible to both scientists and home enthusiasts alike. In the laboratory, decantation is often used as an initial step to separate liquids with different densities. For instance, when separating a mixture of water and an organic solvent, decantation can quickly remove the majority of the water, leaving the organic solvent behind for further processing.
Beyond the lab, decantation plays a crucial role in industries such as food and beverage production. In winemaking, for example, decanting helps remove sediment and other impurities from the wine, resulting in a clearer, smoother product. Similarly, in oil refineries, decantation is employed to separate water and other impurities from crude oil, ensuring the purity of the final product.
Related Methods:
Centrifugation
While decantation relies on gravity to separate liquids, centrifugation employs centrifugal force to accelerate the process. In this technique, the liquid mixture is placed in a centrifuge, a device that spins rapidly, creating a strong centrifugal force that pulls the denser liquid outward. This force speeds up the separation, allowing for more efficient and precise separation of liquids with similar densities.
Extraction
Extraction is another technique that leverages differences in solubility to separate substances. In this process, a solvent, typically a liquid, is added to the mixture, which selectively dissolves one component of the mixture. The dissolved component can then be separated from the rest of the mixture by decantation or other separation methods.
**Centrifugation: A Whirlwind of Separation**
Imagine a spinning vortex, its centrifugal force drawing particles apart like a cosmic dance. This is the essence of centrifugation, a technique that harnesses the power of rotation to **separate substances based on their density and size**. From towering laboratory centrifuges to the tiny handheld models used in medicine, this invaluable method plays a crucial role in a multitude of scientific and industrial endeavors.
Centrifugation operates on the principle of **sedimentation**. When a suspension of particles is **spun rapidly**, the denser particles experience a greater centrifugal force and are hurled outward, settling at the bottom of the container. This sedimentation process is influenced by several factors, including the particle size, density difference between the particles and the suspending medium, and the rotational speed.
One striking application of centrifugation is in the field of **biochemistry**. By spinning blood samples, scientists can **separate red blood cells, white blood cells, and plasma**, each with its unique density. This **fractionation** enables researchers to study the composition and function of these components, aiding in the diagnosis and treatment of diseases.
In the realm of chemistry, centrifugation is employed to **purify and isolate compounds**. For instance, in the synthesis of antibiotics, centrifugation can **remove unwanted byproducts and impurities**, resulting in a purer final product. It also finds use in the **extraction of DNA from cells**, allowing for genetic analysis and medical diagnostics.
Centrifugation’s versatility extends to **industrial applications**. In the food industry, it is used to **clarify fruit juices, separate milk components**, and **concentrate enzymes**. In the environmental sector, it **removes pollutants and contaminants from water and wastewater**. Its applications are truly diverse, from **separating uranium isotopes** to **purifying biodiesel**.
To enhance the separation efficiency of centrifugation, related techniques like **filtration** and **chromatography** can be employed in conjunction. Filtration **removes larger particles** before centrifugation, while chromatography **separates substances based on their chemical affinities**. This combination of techniques allows for **precise and selective separation**, making centrifugation an indispensable tool in countless scientific and industrial settings.
Chromatography: Unraveling the Secrets of Molecular Separation
In the world of chemistry, chromatography stands as an indispensable technique for separating substances based on their intricate dance with different phases. Picture a journey where molecules embark on a quest through a maze, their progress dictated by their affinity for the stationary and mobile phases that guide their path.
Imagine a bed of tiny, inert particles that form the stationary phase, patiently waiting to greet the incoming molecules. These particles could be anything from sand to sophisticated porous materials like silica gel. Meanwhile, a mobile phase, liquid or gas in nature, acts as a gentle escort, carrying the molecules through the maze.
As the mobile phase trickles through the stationary phase, a fascinating game of “musical chairs” ensues. Molecules that have a stronger attraction to the stationary phase linger longer, while those with less affinity zip right through. This difference in preference creates a separation effect, with molecules of varying affinities partitioning themselves along the length of the maze.
Chromatography finds its home in various fields. In analytical chemistry, it’s used to identify and quantify compounds in complex mixtures, such as those found in food, drugs, and environmental samples. In preparative chemistry, it’s employed to purify desired substances for use in pharmaceuticals, cosmetics, and industrial products.
Chromatographic techniques are surprisingly diverse, each with its unique strengths. Paper chromatography, the simplest form, uses a strip of paper as the stationary phase. Thin-layer chromatography (TLC) offers a step up, using a thin layer of adsorbent material spread on a glass or plastic plate.
Gas chromatography (GC) and liquid chromatography (LC) represent more sophisticated methods. GC separates volatile compounds based on their affinity for a gas phase, while LC relies on a liquid mobile phase. HPLC (High-Performance Liquid Chromatography) and UPLC (Ultra-High-Performance Liquid Chromatography) are particularly powerful LC variations, enabling rapid and precise separations of complex mixtures.
The applications of chromatography extend far beyond the laboratory. In the medical field, it’s used to diagnose diseases by analyzing blood, urine, and tissue samples. In environmental science, it plays a crucial role in monitoring pollution levels and assessing the safety of water, air, and soil.
In essence, chromatography is a powerful tool that allows scientists and researchers to delve into the molecular world, separating substances with precision and unlocking the secrets of their chemical compositions.
Distillation:
- Describe the principle and application of separating liquids with different boiling points, including related techniques like sublimation and chromatography.
Distillation: A Method for Separating Liquids
In the world of chemistry and industry, the ability to separate substances is crucial. Distillation stands as a versatile technique for isolating liquids based on their differing boiling points. This separation method finds applications in a wide range of fields, including medicine, perfume manufacturing, and even the production of alcoholic beverages.
The Essence of Distillation
The principle behind distillation is relatively straightforward. When a liquid mixture is heated, the components with lower boiling points will begin to vaporize at a faster rate than those with higher boiling points. By carefully controlling the temperature and collecting the vapors, chemists can effectively separate the individual components.
The Process Unraveled
In a typical distillation setup, the liquid mixture is heated in a flask equipped with a condenser. The condenser cools and condenses the vaporized components, which then collect in a separate container. The condensed liquids can then be further purified through repeated distillations.
Applications in Diverse Industries
- Medicine: Distillation is used to purify pharmaceuticals and isolate essential oils for medicinal purposes.
- Perfume Manufacturing: This technique plays a crucial role in extracting fragrant compounds from flowers and other natural sources.
- Alcoholic Beverage Production: Distillation is essential for separating ethanol from water in the production of spirits such as whiskey and vodka.
Related Techniques
- Sublimation: This method involves directly converting a solid into a gas, bypassing the liquid phase. It is often used to purify substances that decompose at high temperatures.
- Chromatography: A separation technique that utilizes the differential migration of molecules between a stationary and a mobile phase. Distillation can be combined with chromatography for further purification.
Sublimation: A Journey from Solid to Gas without Liquid Interlude
In the world of substance separation, sublimation stands out as a captivating process that defies the ordinary path from solid to liquid to gas. This unique transformation allows solids to skip the liquid phase altogether, embarking on a direct ascent to the gaseous realm.
Imagine a solid substance, its particles tightly packed together. As heat intensifies, these particles begin to break free from their crystalline confines, soaring into the air as gaseous molecules. Like ethereal spirits, they dance freely, casting aside their solid form.
This phenomenon finds practical application in various fields. Take freeze-drying, where delicate substances like food and pharmaceuticals are preserved by removing water through sublimation. The frozen material is placed in a vacuum chamber, where the ice crystals bypass the liquid stage and transform directly into water vapor, leaving behind a dry, shelf-stable product.
Purification of solids is another area where sublimation excels. By carefully controlling temperature and pressure, impurities can be separated from the desired solid. As the solid sublimates, the impurities remain behind, leaving behind a purer substance.
Moreover, sublimation plays a crucial role in crystal growth. By carefully controlling the rate of sublimation and deposition, scientists can create crystals with precise shapes and sizes, essential for various technological applications, such as optics and electronics.
In the realm of scientific analysis, sublimation is employed in the identification of compounds. By observing the temperature at which a substance sublimates, scientists can gain valuable insights into its molecular structure and composition.
Sublimation, with its ability to bypass the liquid phase, stands as a testament to the versatility and power of substance separation techniques. From preserving delicate foods to purifying materials and crafting intricate crystals, sublimation continues to inspire innovation and advance scientific frontiers.
Crystallization: A Journey to Separate the Purest Solids
In the realm of substance separation, crystallization takes center stage as an elegant and transformative process. It’s a journey that leads us from impure solutions to pristine solids, revealing the hidden treasures within.
Crystallization begins with a supersaturated solution, where the solvent holds more dissolved substance than it can normally accommodate at a given temperature. As we cool the solution or evaporate the solvent, a nucleation event occurs, marking the birth of tiny crystals. These microscopic seeds serve as the foundation upon which the pure solid will grow.
Time and temperature play a crucial role in crystallization. Slow cooling and controlled evaporation allow the crystals to grow steadily and achieve their thermodynamic equilibrium shape. This process ensures that the crystals develop well-defined facets and a uniform size distribution.
Various techniques enhance the crystallization process. Sublimation involves directly converting solids into gases, bypassing the liquid phase. Chromatography helps purify substances by separating them based on their different affinities for a stationary and a mobile phase.
The applications of crystallization are vast. It’s essential in purifying metals, pharmaceuticals, and organic compounds. In the food industry, it’s used to extract sugar from sugarcane and salt from seawater. And in the chemical industry, it plays a pivotal role in synthesizing new materials with tailored properties.
In essence, crystallization is an art form, transforming impure solutions into the crystalline essence of pure substances. It’s a testament to the power of science to unravel the complexities of the natural world and to harness them for human benefit.
Extraction: The Art of Separating Substances Using Solvents
In the realm of substance separation, extraction stands out as a technique that relies on the principle of solubility. It’s a process that involves separating substances based on their varying levels of solubility in different solvents.
Imagine a mixture of oil and water. These two liquids, being immiscible (or, in other words, not able to mix), form distinct layers. When a separatory funnel is used to introduce a suitable solvent – such as hexane – the oil will dissolve into the hexane, creating a separate layer on top of the water.
The underlying principle of extraction lies in the affinity of a solute (the substance to be separated) for different solvents. The solvent that best dissolves the solute will selectively extract it from the mixture.
Chromatography and magnetic separation are techniques closely related to extraction. Chromatography involves separating substances by passing them through a stationary phase (a solid or liquid) and a mobile phase (a liquid or gas). Substances with different affinities for the stationary phase will move at different rates, enabling their separation.
Magnetic separation employs magnets to isolate magnetic materials from non-magnetic ones. This technique finds applications in industries like mining and waste recycling.
Extraction plays a crucial role in various scientific and industrial processes. It’s used in the isolation of natural products, pharmaceutical drugs, and metals. In the food industry, extraction is employed to obtain essential oils and flavors. It also finds applications in environmental analysis, where it helps detect and measure pollutants in soil and water samples.
In essence, extraction empowers scientists and industries alike with the means to selectively isolate substances based on their solubility. Its versatility and wide-ranging applications underscore its significance in the pursuit of scientific knowledge and industrial advancements.
Magnetic Separation: A Forceful Way to Separate Materials
In the realm of substance separation, magnetic separation stands out as a technique that harnesses the power of magnetism to segregate materials based on their magnetic properties. This technique finds applications in diverse fields, ranging from mining and recycling to medical diagnostics and environmental remediation.
Magnetic separation relies on the simple principle that magnetic materials are attracted to magnets, while non-magnetic materials are not. This difference in attraction allows for the selective removal of magnetic particles from a mixture. The process involves passing the mixture through a magnetic field, causing the magnetic particles to adhere to the magnet while the non-magnetic particles continue to flow through.
Applications of Magnetic Separation
The versatility of magnetic separation extends to various industries and applications, including:
- Mining: Magnetic separation is employed to extract valuable magnetic minerals, such as iron ore and magnetite, from raw materials.
- Recycling: This technique is used to separate ferrous metals from non-ferrous metals in electronic waste and other recyclable materials.
- Medical diagnostics: Magnetic beads coated with specific antibodies are used to isolate target cells or molecules in blood samples for analysis.
- Environmental remediation: Magnetic particles can be used to remove heavy metals and other contaminants from water and soil.
Related Techniques
Magnetic separation often complements other separation techniques, such as:
- Filtration: Magnetic filtration systems use magnets to collect magnetic particles from fluids, enhancing the efficiency of liquid purification.
- Decantation: In some cases, magnetic separation is combined with decantation to separate magnetic solids from liquids, allowing for the removal of non-magnetic impurities.
Advantages of Magnetic Separation
Magnetic separation offers several advantages:
- High selectivity: Magnetic separation provides precise and efficient segregation of magnetic materials from non-magnetic materials.
- Scalability: This technique can be applied to both small-scale and large-scale operations, making it suitable for industrial applications.
- Environmental friendliness: Magnetic separation does not involve the use of harsh chemicals or solvents, reducing the environmental impact.
Magnetic separation is a powerful technique that leverages magnetism to separate materials based on their magnetic properties. Its versatility and efficiency make it a valuable tool in various industries, from mining and recycling to healthcare and environmental protection. By harnessing the force of magnets, magnetic separation continues to contribute to advancements in material science and beyond.