Acetic Acid Molar Mass: Calculating The Molecular Weight Of Ch3Cooh
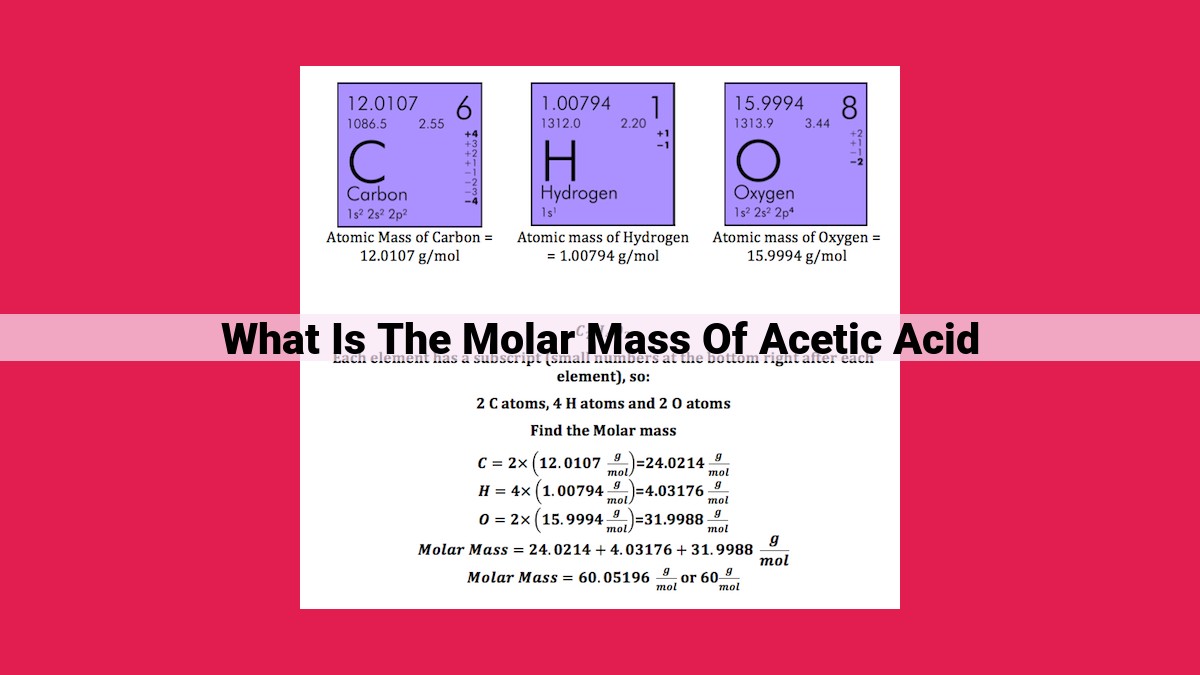
Molar mass of a substance is its mass per mole, a fundamental unit in chemistry. To calculate the molar mass of acetic acid (CH3COOH), also known as ethanoic acid, we add the molar masses of its constituent elements: carbon (C), hydrogen (H), and oxygen (O). Using the periodic table, we find that each carbon atom contributes 12.01 g/mol, each hydrogen atom 1.01 g/mol, and each oxygen atom 16.00 g/mol. Doubling the oxygen contribution due to two oxygen atoms in the molecule, we get the molar mass of acetic acid as 60.05 g/mol. Understanding molar mass enables us to determine the amount of substance present, perform stoichiometric conversions, and gain insights into the molecular composition of compounds like acetic acid.
Understanding Molar Mass: A Key to Unlocking Chemistry
In the realm of chemistry, understanding the concept of molar mass is akin to having a magic wand that unlocks the mysteries of matter. It’s the fundamental quantity that allows us to bridge the gap between the microscopic world of atoms and molecules and the macroscopic world we experience.
Molar mass represents the mass of one mole of a substance. A mole, itself, is a colossal number: 6.022 × 10^23 units. Imagine a vast army of atoms or molecules, so numerous that it would take eons to count them individually. That’s the power of a mole.
Understanding Acetic Acid: The Tangy Component of Vinegar
In the culinary world, vinegar stands as a pantry staple, adding a delightful acidity to dressings, marinades, and preserves. But what lies at the heart of vinegar’s tangy essence? Enter acetic acid, a colorless liquid with a sharp, pungent odor.
Acetic acid, also known by its chemical name ethanoic acid, has the molecular formula CH3COOH. It’s a weak acid, meaning it doesn’t completely dissociate into ions in water. This unique property contributes to vinegar’s mildness compared to stronger acids like hydrochloric acid.
Acetic acid’s presence in vinegar is a result of the fermentation process. When certain bacteria encounter ethanol (alcohol), they convert it into acetic acid, giving rise to the characteristic sour flavor of vinegar.
Understanding Mass, Mole, and Avogadro’s Number
In the realm of chemistry, precision is paramount. Two crucial concepts that underpin this precision are mass and mole.
Mass quantifies the matter an object contains, while a mole represents a specific amount of a substance. Imagine a grocery store where each apple weighs approximately 100 grams. If you buy 10 apples, you’re purchasing 1000 grams of apples. Similarly, in chemistry, if you have 1 mole of a substance, you’re handling a specific number of its particles (atoms, molecules, ions, etc.).
The connection between mass and mole is established through Avogadro’s number, which is a fundamental constant. It represents the number of particles present in exactly 1 mole of any substance. This number is a staggering 6.022 × 10^23, akin to counting the grains of sand on all the beaches on Earth. Avogadro’s number allows us to bridge the gap between mass and amount, enabling accurate chemical calculations.
Calculating the Molar Mass of Acetic Acid: A Chemical Adventure
In Chemistry, we often encounter terms like molar mass, which play a crucial role in understanding the composition and behavior of substances. Let’s delve into the fascinating journey of calculating the molar mass of acetic acid, a common ingredient in vinegar.
Deciphering the Molar Mass Formula
The molar mass of any substance is the sum of the atomic masses of all the elements it contains. The formula for calculating the molar mass is:
Molar Mass = Mass of Element 1 + Mass of Element 2 + ...
For acetic acid, we have the chemical formula CH3COOH. This means it contains carbon, hydrogen, and oxygen atoms.
Determining the Molar Mass of Each Element
To calculate the molar mass of acetic acid, we need to know the molar mass of each element. We can find these values from the periodic table:
- Carbon (C): 12.011 atomic mass units (amu)
- Hydrogen (H): 1.008 amu
- Oxygen (O): 16.000 amu
Performing the Calculation
Now, we can plug the molar masses of each element into the formula for acetic acid:
Molar Mass of CH3COOH = Molar Mass of C + 3 × Molar Mass of H + 2 × Molar Mass of O
= 12.011 amu + 3 × 1.008 amu + 2 × 16.000 amu
= 60.052 amu
Therefore, the molar mass of acetic acid is 60.052 amu.
Significance of Molar Mass
Understanding the molar mass of acetic acid is essential for various chemical calculations. It helps us:
- Determine the exact amount of substance present in a given mass.
- Perform stoichiometric conversions between different reactants and products.
- Gain insights into the substance’s molecular composition.
By grasping the concept of molar mass, we unlock the secrets of chemical reactions and deepen our understanding of the molecular world.